Philadelphia Health Officials Prepare For Child Vaccinations As Pfizer Seeks FDA Authorization For Children Under 12
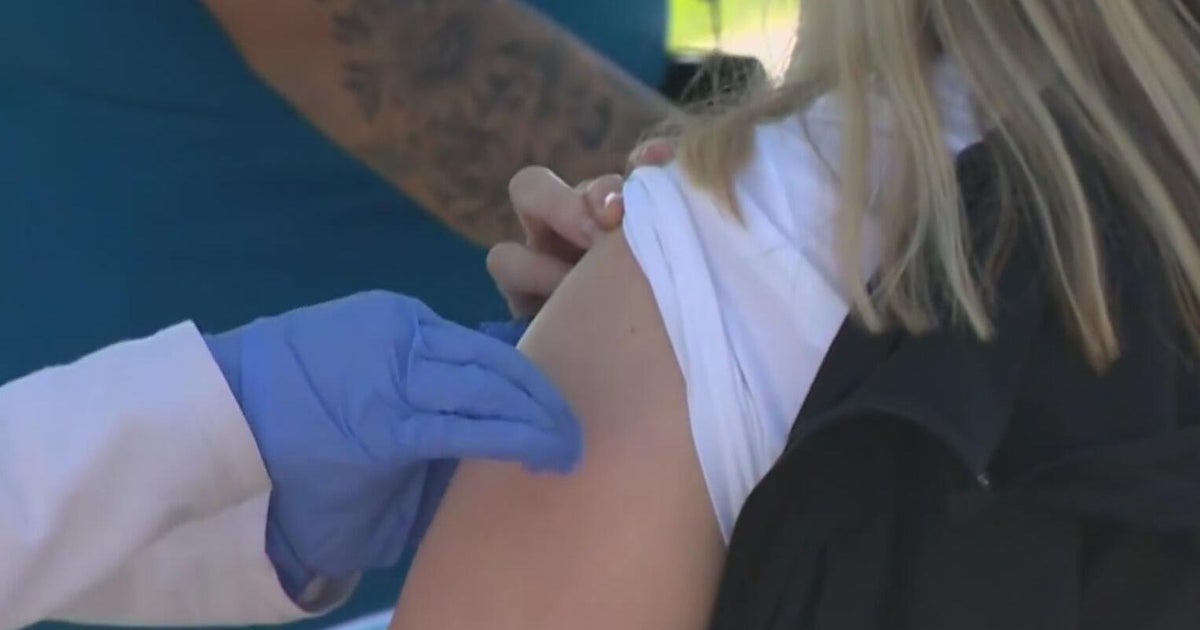
PHILADELPHIA (CBS) -- There was a big step towards getting COVID-19 vaccines for younger children on Thursday. Pfizer officially applied for emergency use authorization for its vaccine for children ages 5- to 11-years-old.
The request comes as Pfizer and BioNTech say the research shows younger children should get a third of the dose that has been given to those 12-years-old and older.
If this clears federal regulators, expanding the use of the vaccine will help combat the alarming increase in COVID-19 cases among children and help schools stay open.
The FDA will review Pfizer research that says its vaccine is safe and effective for children between the ages of 5 to 11. It's currently only cleared for those 12 and above with full protection coming after two doses.
But for younger children, there's one big change. Pfizer recommends they get one-third of the dose given to everyone else.
Former FDA Commissioner Dr. Scott Gottlieb is a member of Pfizer's Board of Directors.
"Pfizer tested multiple doses to find the optimal dose, that had the best compromise between reducing the number of vaccine-related side effects while still providing efficacy that is on par with what we saw in 16- to 25-year-olds," Gottlieb said.
Pfizer says in trials more than 2,200 children were given one-third of the dose adults get. The company found its vaccine is safe and generated a robust antibody response.
"We are ready. We have the supply. We're working with states to set up convenient locations for parents and kids to get vaccinated including pediatrician's offices and community sites," White House COVID-19 Response Coordinator Jeff Zients said.
About 28 million American children are between the ages of 5 to 11 and will be eligible for the vaccine if it's cleared.
"I would anticipate that as parents and children have questions with their pediatricians and other local health officials and they get those questions answered that more and more parents and kids will want to get their kids vaccinated," Zients said.
Dr. Julia Sammons of the Children's Hospital of Philadelphia told CBS3 the news is a long time coming.
"I think this is news we've been waiting for," she said.
Pfizer studied the lower dosage in more than 2,200 volunteers and said there were no serious side effects. According to Dr. Sammons, the smaller dosage is due to younger children having a stronger immune system.
"It will be the same vaccine," she said, adding, "[It's] not necessarily due to their smaller size but due to the fact that children have more robust immune systems, so it frequently takes less dose to elicit that same robust response."
Pfizer has said the plan is to have special-marked pediatric vials of the vaccine. The FDA has an emergency meeting scheduled for Oct. 26, with a decision expected between Halloween and Thanksgiving.