Moderna Seeks Emergency Use Authorization For COVID-19 Vaccine For Children Ages 6 Months Through 5 Years
PHILADELPHIA (CBS) -- A COVID-19 vaccine for young children could be on the way. Moderna on Thursday submitted a request to the U.S. Food and Drug Administration for emergency use authorization for its vaccine in children under the age of 6.
Moderna says its vaccine is safe and effective for young children. It's something millions of families are waiting for. The FDA says making a vaccine available for kids younger than 5 is one of its highest priorities.
Theo is one of the thousands who tested the Moderna vaccine along with his twin brother.
"We wanted them vaccinated as soon as possible and we also just very much wanted to see our vaccine trials move forward so that this youngest group of kids could also get protected," mom Anne Rodriguez said.
The most recent research from Moderna shows that two doses of its vaccine have a 51% efficacy rate for children 6 months to 2 years old and a 37% efficacy rate for children 2 to under 6 years old.
"People can be reassured and confident that these kids will get safely protected against disease," said Dr. Paul Burton, Moderna's chief medical officer.
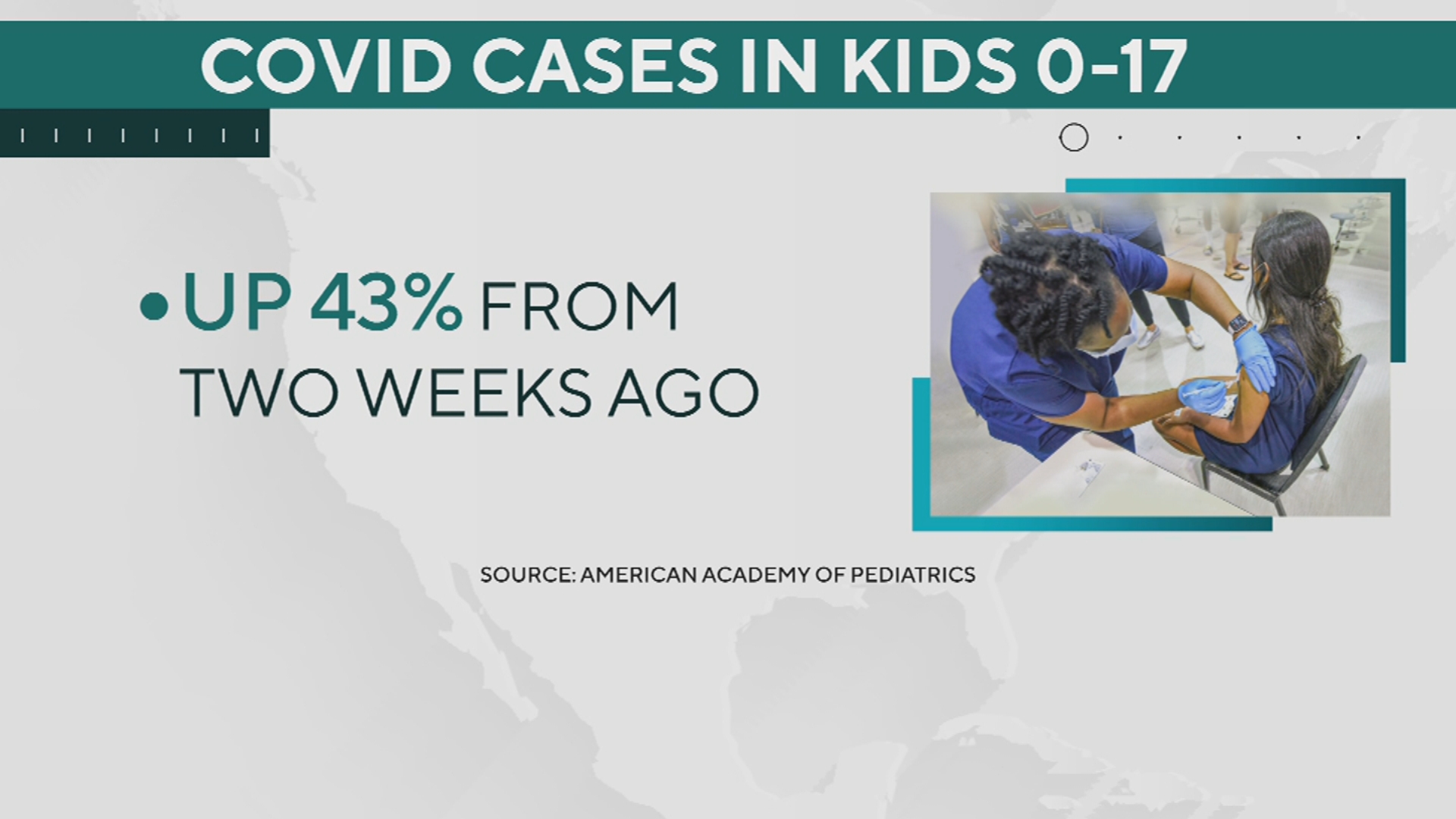
Moderna's research comes as COVID cases in kids are climbing, up 43% from two weeks ago.
Parents with young children have been anxiously waiting for the clearance of a vaccine.
Currently, only children over the age of 5 can receive the Pfizer vaccine. That leaves about 18 million youngsters unprotected.
"The last two years have been nothing that we would have ever imagined," Rodriguez said. "Having vaccines for these youngest kids feels like a light end of the tunnel."
Moderna said the most common reactions to its vaccine in young children were pain at the injection site and fever.
The FDA said Thursday it will schedule a meeting to review Moderna's research after receiving more data from the company.
There's no indication yet on when a final ruling would be made.