Sleep apnea patients left frustrated & waiting as massive Philips CPAP recall drags on
FORT WORTH (CBSNewsTexas.com) — A massive recall of millions of sleep apnea machines that has dragged on for nearly two years has left many patients waiting with seemingly no good options.
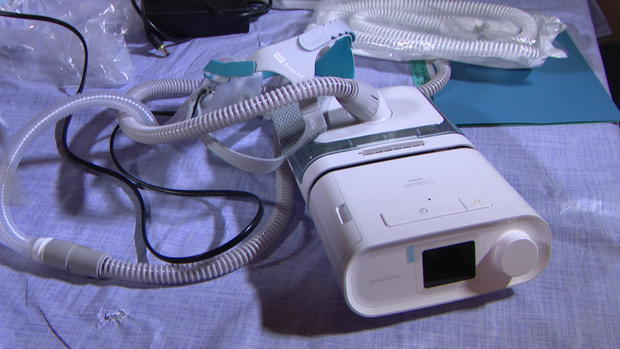
In June of 2021, Philips Respironics issued a recall of more than 5-million breathing devices.
The company warned a foam, meant to reduce noise, was breaking off and blowing into the mouths of users. Inhaling foam particles, the company wrote in a 2021 notice, could result in "serious injury which can be life-threatening."
Philips Respironics, which produces more than 40% of the world's sleep apnea devices, initially estimated it could replace all effected devices within a year. However, more than a year and a half later, many patients are still waiting.
"It has been horrible," said Janet Gray of White Settlement, whose Philips Respironics DreamStation CPAP machine was one of the models recalled.
"You are afraid to go to sleep because if you go to sleep without it, you might die. But if you go to sleep with it, it might kill you down the road," she said.
Philips Respironics said it has now completed production on 90% of the devices needed for the original recall.
Gray received a refurbished replacement device nearly 18 months after the recall. The foam inside had been pulled out and replaced with silicone.
However, Gray said she will not be using it.
"I don't trust it," she said. "I trust it less than the first one."
Since April of 2021, the U.S. Food and Drug Administration (FDA) has received 98,000 complaints associated with the foam breakdown in Philips Respironics' beathing devices. Complaints included infections, chest pain and cancer. There were also 346 reports of death. The FDA said these reports are helpful in identifying problems but because they are self-reported, they lack verification.
Philips Respironics said it found "no conclusive data linking these devices and the deaths reported." A company spokesperson told the I-Team, after the 2021 recall, it commissioned additional testing and found no association between its devices and cancer.
The FDA called Philips Respironics' new analysis "unpersuasive."
Dr. Vikas Jain of Frisco's Dream Sleep Medicine clinic said the lack of communication from Philips Respironics about the recall put physicians in a tough spot. The board-certified sleep doctor said he still has new patients coming in unaware their devices have been recalled. With no clear timetable on when patients will receive a replacement, Jain said it's been extremely difficult to provide patients with sound advice.
"For the company to come to us and say, 'We don't know when we are going to be able to send a replacement, but you should talk to the patient and decide whether or not they should continue to use the device...' that's a tough spot," the Frisco doctor said. "I'm supposed to decide if a patient is supposed to use a device that may cause cancer or not use it at all. That is going to be the burden you place on physicians without having a clear process in place."
As doctors struggle with what to tell patients, shortly after the recall, the FDA conducted an inspection of a Philips Respironics manufacturing facility in Pennsylvania.
In the report, federal inspectors noted Philips Respironics knew there was problem with the foam in its breathing devices in 2015— six years before the recall. Yet, according the FDA inspection report, no additional design change or corrective action was taken.
A Philips Respironics spokesperson told the I-Team, "In prior years, there were limited complaints related to foam degradation, which [Philips Respironics] evaluated and addressed on a case-by-case basis. Potential concerns relating to the emission of volatile organic compounds began to surface only more recently. When Philips became aware of the issue and its potential significance in early 2021, actions were taken leading to the field safety notice in June of 2021."
FDA 2021 Inspection of Philips by CBS 11 News on Scribd
Late last year, Philips Respironics began recalling more than 20,000 refurbished ventilators citing two different issues—leftover foam particles were still found in some of the reworked devices and the silicone that replaced the foam in some cases was coming unglued and could potentially block the airflow. Last month, the FDA marked this as a class one recall, meaning it's the most serious type. The Philips' Trilogy 100 and 200 ventilators were a part of the more than 5 million devices recalled in 2021. This latest recall of repaired devices does not include any of the CPAP devices.
Philips recalled the following devices made between 2009 and April 26, 2021:
- A-Series BiPAP A30
- A-Series BiPAP A40 (ventilator)
- A-Series BiPAP Hybrid A30
- A-Series BiPAP V30 Auto (ventilator)
- C-Series ASV (ventilator)
- C-Series S/T and AVAPS
- DreamStation
- DreamStation ASV
- DreamStation Go
- DreamStation ST, AVAPS
- Dorma 400
- Dorma 500
- E30
- Garbin Plus, Aeris, LifeVent (ventilator)
- OmniLab Advanced+
- REMstar SE Auto
- SystemOne ASV4
- SystemOne (Q-Series)
- Trilogy 100 (ventilator)
- Trilogy 200 (ventilator)
Philips also recalled certain Trilogy Evo ventilators distributed from April 15, 2021, to May 24, 2021, with specific serial numbers.
If you use a recall device, the FDA recommends the following:
- Do not stop or change ventilator use until you have talked to your health care provider.
- Talk with your health care provider about using an inline bacterial filter, which may help to filter out pieces of PE-PUR foam, as indicated in the Philips recall notification. At this time, the information provided by Philips has not established that the filters can reduce the PE-PUR foam's risks. The FDA's evaluation of the information provided by Philips is ongoing. It is important to note the following considerations: 1) Inline bacterial filters will not help to reduce contact with certain chemicals that may be released from the PE-PUR foam. 2) Inline bacterial filters may increase the resistance to air flow through the device, which could mean the ventilator will not ventilate adequately. 3) If your ventilator has an inline bacterial filter, closely monitor for PE-PUR foam pieces collecting on the filter or airflow problems.
- Register your device(s) on Philips' recall website or call 1-877-907-7508 to provide important additional information to help prioritize replacement of your device and to get updated information from Philips. For Spanish translation, press 2; Para español, oprima 2.
- If you have a health issue, including those listed under potential health risks, or any problem with your device, talk to your health care provider and report the issue or problem through the MedWatch Voluntary Reporting Form.