
FDA making plans to end its routine food safety inspections, sources say
Food safety inspections would be left to state and local authorities under the plan being developed by the FDA.
Watch CBS News
Alexander Tin is a digital reporter for CBS News based in the Washington, D.C. bureau. He covers federal public health agencies, including the response to infectious disease outbreaks like COVID-19. Previously, he was a campaign reporter for CBS News based out of Las Vegas, where he was raised. He covered presidential, Senate and House candidates for the 2020 election cycle in Arizona, California, Nevada and New Mexico. He has also worked in Washington for "Face the Nation" and in New York for the "CBS Evening News." Tin graduated from Columbia University in 2017 with a bachelor's degree in political science.

Food safety inspections would be left to state and local authorities under the plan being developed by the FDA.

HHS denied censoring Dr. Kevin Hall, the researcher who announced his early retirement on Wednesday, saying it was a "deliberate distortion of the facts."
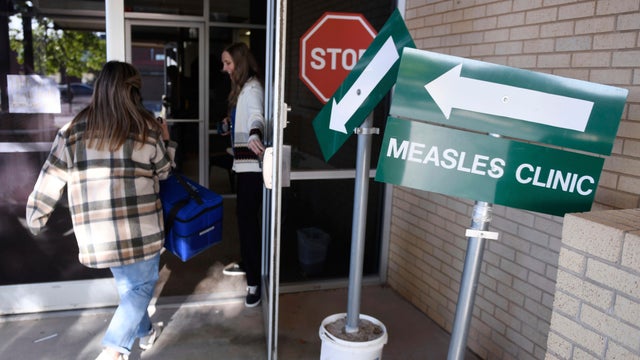
The CDC is now struggling to keep up with requests for support from states with measles outbreaks.
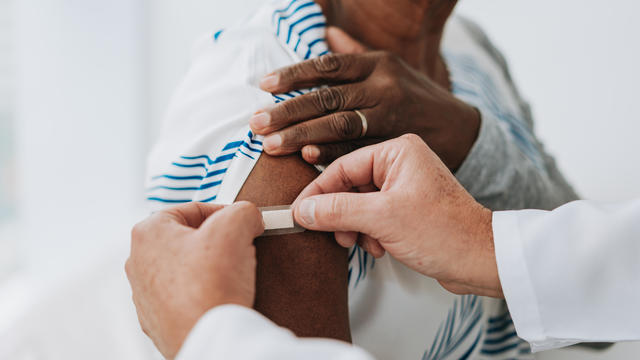
A majority of the agency's COVID-19 vaccine work group now backs narrower "risk-based" recommendations.

CDC experts were not made available to discuss the findings showing a rise in autism prevalence.

Dr. Peter Marks said that the deaths of unvaccinated children is "just not acceptable."

Steep cuts to the agency's workforce had disrupted drug and food safety inspections.

"You can be incredibly supportive of people, but giving them false hope is wrong," said Dr. Peter Marks.

Arkansas, Hawaii and Indiana have joined a list of two dozen states with confirmed measles cases.

The CDC rejected a request for help "due to the complete loss" of their lead poisoning experts.

The steep cuts to the cruise ship inspection team baffled officials in the program, which is not paid for by taxpayer dollars.
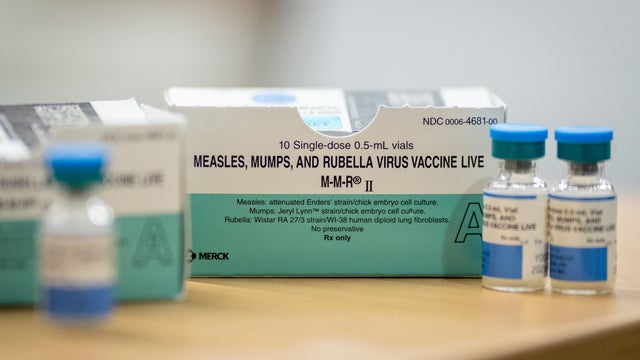
The CDC is now backing an additional measles vaccine shot for some travelers within the United States in response to record outbreaks.

Nearly half of the National Center on Birth Defects and Developmental Disabilities was laid off.

The CDC's team of lead poisoning experts remained off the job a week after the sweeping HHS layoffs.

Kennedy's comment comes as the Environmental Protection Agency says it has now launched a new review of fluoride's health effects.