CDC warns about enterovirus in kids — and risk of rare paralysis
Data from hospitals suggest enterovirus D68, which in rare cases can lead to paralysis in kids, is spreading at its highest levels since 2018.
Watch CBS News
Alexander Tin is a digital reporter for CBS News based in the Washington, D.C. bureau. He covers federal public health agencies, including the response to infectious disease outbreaks like COVID-19. Previously, he was a campaign reporter for CBS News based out of Las Vegas, where he was raised. He covered presidential, Senate and House candidates for the 2020 election cycle in Arizona, California, Nevada and New Mexico. He has also worked in Washington for "Face the Nation" and in New York for the "CBS Evening News." Tin graduated from Columbia University in 2017 with a bachelor's degree in political science.

Data from hospitals suggest enterovirus D68, which in rare cases can lead to paralysis in kids, is spreading at its highest levels since 2018.
Officials in Los Angeles County said autopsy results could be available in "as soon as a few days."
The federal budget for buying and distributing COVID-19 vaccines will run out "as early as January."

The first shots in the Biden administration's fall booster campaign could start in days, after a vote by the CDC's advisers.

The first shots in the Biden administration's fall booster campaign could start in days, after a vote by the CDC's advisers.

Federal health officials say thousands of updated booster shots are already being shipped around the country.

The CDC said the drop in life expectancy was largely driven by the COVID-19 pandemic.

Health authorities are still investigating the case to determine what role monkeypox may have played in the patient's death.

Americans have until Sep. 2 to order their 16 free tests, if they haven't already.

The looming milestone comes as health officials say they have "cautious optimism" that cases may be slowing in the outbreak.

The updated travel guidance is part of a sweeping overhaul to the CDC's pandemic recommendations.

The CDC counts only 17 monkeypox cases in children under 16 years old so far.

The research is one of several efforts by the Biden administration to address long-term symptoms that linger for some patients.
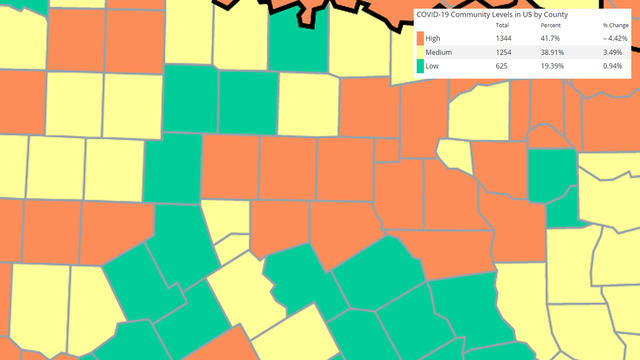
The agency says the new revisions acknowledge "the pandemic is not over" but should not disrupt daily life as much as it used to.

The agency says the new revisions acknowledge "the pandemic is not over" but should not disrupt daily life as much as it used to.