
Updated COVID-19 boosters are rolled out
As fall approaches and COVID-19 immunity wanes, updated boosters target Omicron subvariants. Dr. Celine Gounder explains how the shots work and who should get them right away.
Watch CBS News

As fall approaches and COVID-19 immunity wanes, updated boosters target Omicron subvariants. Dr. Celine Gounder explains how the shots work and who should get them right away.
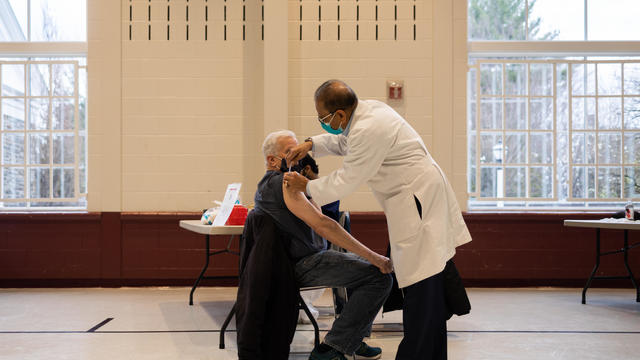
The new shots are rolling out to pharmacies and other vaccination sites around the country.
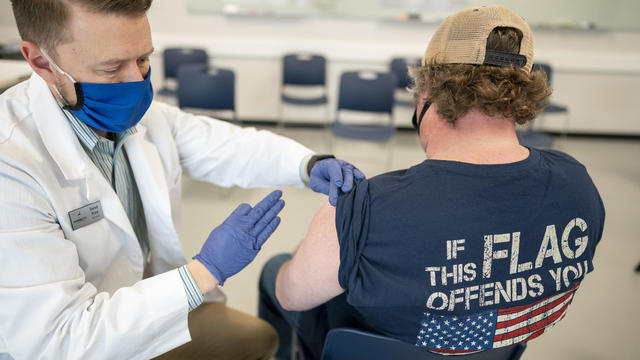
The federal budget for buying and distributing COVID-19 vaccines will run out "as early as January."

Federal health officials say thousands of updated booster shots are already being shipped around the country.

The Food and Drug Administration has authorized new COVID boosters that are designed to be more effective against Omicron subvariants. Dr. David Agus joins CBS News to answer questions from viewers about the vaccine.

New COVID booster shots that target the latest Omicron variants could be available within days, after the FDA authorized the updated boosters made by Pfizer and Moderna.

Two Southern California police officers have been shot and killed. The El Monte officers were investigating a possible stabbing at a motel. The suspect also died at the scene. FDA advisers are recommending that the agency authorize Moderna's COVID vaccine for kids 6 to 17. And Russia has extended WNBA star Brittney Griner's detention until at least July 2.
White House chief medical adviser Dr. Anthony Fauci says the U.S. could see 200,000 new COVID infections a day in the fall. His prediction comes as the Delta variant continues to drive new cases, and as officials race to get more Americans vaccinated. CBS' Skyler Henry reports from the White House. Then, CBS News reporter Alex Tin joins CBSN's Red and Blue host Elaine Quijano to discuss that and more.
The U.S. has reported a decline in COVID-19 vaccination rates for the past 12 days as new cases and hospitalizations rise nationwide. CBS News reporter Alexander Tin joined Elaine Quijano on CBSN to talk the latest on COVID-19 in the U.S.
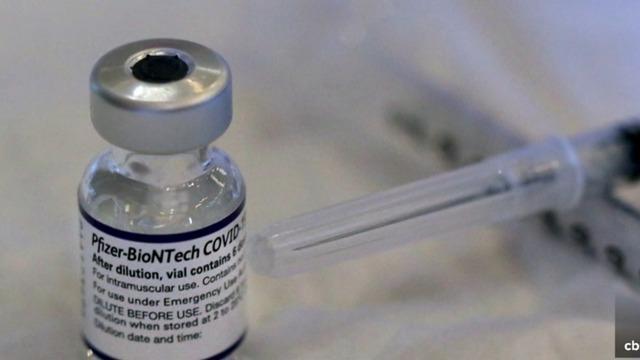
An FDA advisory panel has voted to endorse Pfizer's coronavirus vaccine for children ages 5 to 11 and a final decision is expected soon. CBS News' Debra Alfarone reports, and then CBS News reporter Alex Tin joins CBSN's "Red & Blue" to discuss the latest COVID-19 news.

President Biden is outlining a path forward to increase COVID-19 vaccinations as the highly contagious Delta variant is rapidly gaining a foothold in the U.S. CBS News reporter Alex Tin joins CBSN's "Red & Blue" to discuss the president's plan and the latest on the fight against the pandemic.
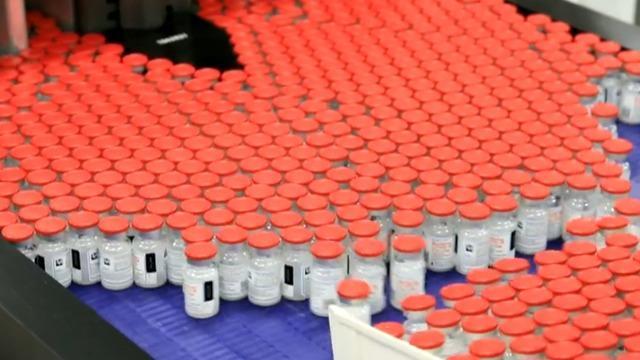
Pfizer and Moderna expect to have updated vaccines to combat the latest Omicron variants as early as September, the Food and Drug Administration confirmed to CBS News.
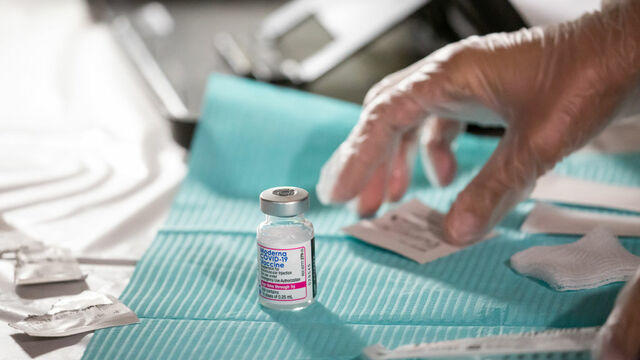
CBS News medical contributor Dr. David Agus joins CBS News' Anne-Marie Green and Vladimir Duthiers to answer all your COVID-related questions including, whether or not you should wait to get boosted if you're taking an antibiotic and if mixing and matching booster doses could give you stronger immunity.

The U.S. Food and Drug Administration is considering ordering a recipe change for the vaccines made by both Pfizer and rival Moderna in hopes that modified boosters could better protect against another surge.

Data shows the booster is highly effective against the virus' Omicron variant, according to the drugmaker

CBS News medical contributor Dr. David Agus joined Anne-Marie Green and Vladimir Duthiers to answer all your questions about COVID-19.

The U.S. has taken another step toward making children younger than 5 years old eligible for COVID vaccinations. Advisers to the FDA on Wednesday unanimously voted to recommend authorization of Pfizer and Moderna's vaccines for kids aged 6 months to under 5 years. It's a critical move for the youngest Americans, who until now have not been able to be inoculated. Meg Oliver reports.
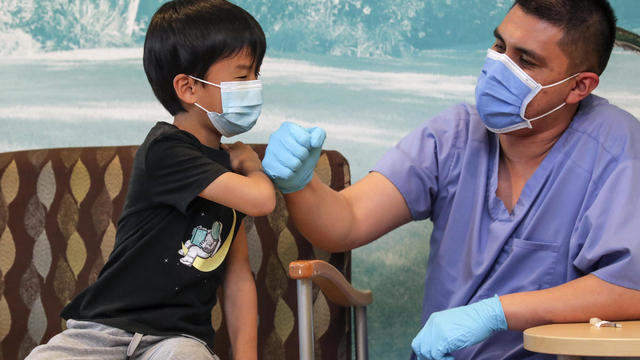
The FDA's panel of vaccine advisers voted unanimously that the benefits of Pfizer's and Moderna's shots outweigh the risks in young children.

The FDA's vaccine advisory panel is meeting today to vote on Pfizer and Moderna's COVID-19 shots for children 5 and under. That same committee voted Tuesday to endorse Moderna's shot for kids ages 6 to 17. CBS News medical contributor Dr. David Agus explains what parents need to know before the FDA and CDC give their final approval.
A panel of advisers to the U.S. Food and Drug Administration is meeting Wednesday to consider approving Pfizer and Moderna's COVID-19 vaccines for young children. Dr. Dyan Hes, founder and medical director of Gramercy Pediatrics in New York City, speaks to "CBS News Mornings" about what parents should know before scheduling vaccination appointments for their kids.
A government advisory panel has endorsed a second brand of COVID-19 vaccine for school-age children and teens.

The White House says doses will be available everywhere from pediatricians' offices to children's museums, if health authorities sign off on the shots later this month.
Moderna says a new version of its COVID-19 vaccine is superior to its original shots and could be available in late summer.

The FDA's vaccine advisers meet later this month to weigh updating booster shots.

Moderna announced Thursday morning that it's asking the FDA to approve its COVID vaccine for kids under 6 years old. If authorized for emergency use, it would become the first eligible vaccine for kids younger than 5. Nancy Chen spoke to Moderna's chief medical officer as well as a parent of two children who participated in the trial.

"Just because it seems impossible to you doesn't mean it's not possible," Vonn says.

The footage is included in a video that promotes false claims that the 2020 presidential election was rigged against Mr. Trump.

Local and federal authorities said "investigators are actively inspecting the information provided in the message for its authenticity" regarding the disappearance of Nancy Guthrie.

With Bad Bunny headlining a historic Super Bowl halftime show, we highlight some of his most impactful lyrics in Spanish and English.

If the June deadline is not met, the Trump administration will likely put pressure on both sides to meet it, Zelenskyy told reporters.

Though the commerce secretary has called his interactions with Epstein as "limited," the two were in business together four years after Epstein's 2008 guilty plea.

Cryptocurrency transactions are often thought to be anonymous and untraceable. That's a misconception, experts tell CBS News.

Hungarian Prime Minister Viktor Orbán attended the launch of the initiative last month in the Swiss ski resort of Davos.

The Pentagon says it will cut ties with Harvard University, ending graduate-level military training, fellowship and certificate programs.

More than three dozen cases of death cap mushroom poisonings have been reported in California since November, health officials said.

If the June deadline is not met, the Trump administration will likely put pressure on both sides to meet it, Zelenskyy told reporters.

The Pentagon says it will cut ties with Harvard University, ending graduate-level military training, fellowship and certificate programs.
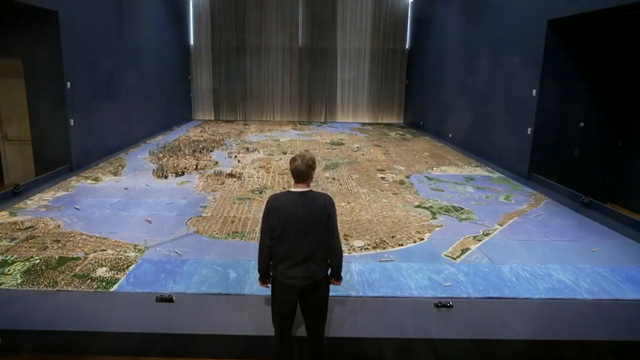
Beginning in 2004, Joe Macken carved all five boroughs of New York City out of balsa wood, every site and stadium, and every bridge and building. His creation consists of almost 1 million structures.

More than 35 local, state and federal agencies have been working for the last 18 months to prepare for Super Bowl LX in Santa Clara, California.

Though the commerce secretary has called his interactions with Epstein as "limited," the two were in business together four years after Epstein's 2008 guilty plea.

Resurgent technology stocks drove the rebound after a volatile week, while bitcoin also recouped losses.

Cryptocurrency transactions are often thought to be anonymous and untraceable. That's a misconception, experts tell CBS News.

Emboldened by loosened restrictions from federal regulators, prediction markets look to cash in on Super Bowl Sunday.

Here's what to know about TrumpRx, including how it works, who can use it, and how much money it can save.

Skier Chris Lillis said he was "heartbroken about what's happened in the United States," while skater Amber Glenn said she "will not just be quiet."

If the June deadline is not met, the Trump administration will likely put pressure on both sides to meet it, Zelenskyy told reporters.

A federal appeals court on Friday endorsed the Trump administration's policy of holding broad groups of immigration detainees without access to bond hearings, a major legal victory for President Trump.

The Pentagon says it will cut ties with Harvard University, ending graduate-level military training, fellowship and certificate programs.

President Trump late Friday addressed a video posted to his social media account that included a racist depiction of Barack and Michelle Obama as apes, telling reporters he didn't see the part that showed the former president and first lady.

Becca Valle, then 37, enrolled in a cutting-edge clinical trial after surgery removed an aggressive tumor from her brain.

More than three dozen cases of death cap mushroom poisonings have been reported in California since November, health officials said.

Here's what to know about TrumpRx, including how it works, who can use it, and how much money it can save.

The Trump administration launched its new TrumpRx direct-to-consumer prescription drug listing site late Thursday, part of a push to offer medication at steep discounts.

The New Mexico Department of Health said officials believe the baby contracted listeria after their mother drank raw milk during pregnancy.

Hungarian Prime Minister Viktor Orbán attended the launch of the initiative last month in the Swiss ski resort of Davos.

Gu qualified for the women's slopestyle final wearing an outfit with details inspired by her Chinese heritage and her personal quirks.

Skier Chris Lillis said he was "heartbroken about what's happened in the United States," while skater Amber Glenn said she "will not just be quiet."

The second gold medal of the Milano Cortina Games was awarded to Frida Karlsson of Sweden in the women's 10km+10km skiathlon.

"Just because it seems impossible to you doesn't mean it's not possible," Vonn says.

Montreal-based brothers Andrew and Brad Barr released their debut album in 2010 and have won some of Canada's top music awards for their indie sound. Performing from their first album in eight years, "Let it Hiss," here's The Barr Brothers performing "Another Tangerine."

Montreal-based brothers Andrew and Brad Barr released their debut album in 2010 and have won some of Canada's top music awards for their indie sound. Performing from their first album in eight years, "Let it Hiss," here's The Barr Brothers performing "Naturally."

Montreal-based brothers Andrew and Brad Barr released their debut album in 2010 and have won some of Canada's top music awards for their indie sound. Performing from their first album in eight years, "Let it Hiss," here's The Barr Brothers performing "Run Right Into It."

Gu qualified for the women's slopestyle final wearing an outfit with details inspired by her Chinese heritage and her personal quirks.

With Bad Bunny headlining a historic Super Bowl halftime show, we highlight some of his most impactful lyrics in Spanish and English.

The FAA says it is collaborating with the FBI to detect, track and assess unauthorized drone activity at the Super Bowl.

Gamers across the world can now recreate drone strikes in Ukraine from the comfort of their own home, with this newly released game.

From labor shortages to environmental impacts, farmers are looking to AI to help revolutionize the agriculture industry. One California startup, Farm-ng, is tapping into the power of AI and robotics to perform a wide range of tasks, including seeding, weeding and harvesting.

CBS News business analyst Jill Schlesinger talks about how companies are using artificial intelligence, the discussion around the technology and how it's impacting the workforce.

Executives from Waymo and Tesla defended their self-driving vehicle technology in testimony before the Senate Commerce Committee on Wednesday. CBS News' Kris Van Cleave reports and Ian Krietzberg, an AI correspondent at the digital media company Puck, has more.

After decades monitoring polar bears in Norway's far north, researchers say the animals have proven incredibly adaptable, but there are no guarantees for the future.

Dark matter doesn't absorb or give off light so scientists can't study it directly. But they can observe how its gravity warps and bends the star stuff around it.

"CBS Saturday Morning" learns more about Veronika, the clever cow who figured out multiple ways to scratch herself with a broom. It was the first time a cow was seen using a tool.
"Sunday Morning" looks back at historical events on this date.

The Dinosaur National Monument, which is located on the border between Colorado and Utah, was last excavated in 1924.

Authorities said Friday they were inspecting an apparent new message relating to the disappearance of "Today" host Savannah Guthrie's mom, Nancy, after the family reported her missing from her home on Sunday.

Luigi Mangione had an outburst after a hearing on Friday in which the judge announced that his New York State trial will begin on June 8. CBS News legal reporter Katrina Kaufman is following the case.

Local and federal authorities said "investigators are actively inspecting the information provided in the message for its authenticity" regarding the disappearance of Nancy Guthrie.

Friday marked six days since Nancy Guthrie's apparent abduction, and Guthrie's three children have been posting on social media hoping to reach whoever may have taken her. CBS News' Andres Gutierrez reports and former FBI counterintelligence operative Eric O'Neill has more.

Luigi Mangione had an outburst in a New York courtroom on Friday after a judge scheduled his state trial to begin before his federal case. The UnitedHealthCare CEO murder suspect claimed "this is the same trial twice" and called it "double jeopardy." CBS News' Katrina Kaufman has more.

NASA's first crewed moon mission in more than 50 years has been delayed until March at the earliest. During a routine dress rehearsal of the launch, persistent liquid hydrogen leaks were discovered in the Artemis II rocket. CBS News space consultant Bill Harwood breaks it down.

NASA plans to test the planned leak repair with a second dress rehearsal fueling test later this month.

NASA delayed the Artemis II moon rocket launch after a hydrogen leak was found during a wet dress rehearsal, the agency announced Tuesday. CBS News senior space consultant Bill Harwood has the latest.

A NASA mission is underway to map the heliosphere, which is a huge protective bubble around the solar system that was created by the sun.

NASA says it can't try until March at the earliest to send a crewed spacecraft on a flight around the moon and back, due to hydrogen leaks during testing of the Artemis II rocket.

A look back at the esteemed personalities who've left us this year, who'd touched us with their innovation, creativity and humanity.

Does the evidence show a cover-up, or was Todd Kendhammer wrongfully convicted for the murder of his wife?

Christy Salters-Martin dominated in the boxing ring but faced her toughest challenger at home.

Family seeks answers in death of newlywed who disappeared in 2005 while on Mediterranean honeymoon cruise.

Meet the tattooed beauty charged in the death of Google executive Forrest Hayes.

"CBS Saturday Morning" explores Northern Italy and breaks down the multiple locations of the Milano Cortina Winter Olympics.

On this edition of CBS Mornings Deals, we show you items that might just become essentials in your everyday life. Visit cbsdeals.com to take advantage of these exclusive deals today. CBS earns commissions on purchases made through cbsdeals.com.

"CBS Saturday Morning" dives into ways people can raise and maintain their credit score.

President Trump is blaming a staffer for the now-removed social media post that included a racist video of former President Barack Obama and former first lady Michelle Obama depicted as apes. Mr. Trump told reporters he is "the least racist president you've had in a long time."

New England Patriots owner Robert Kraft's Blue Square Alliance Against Hate was launched in 2025, following the success of his 2023 Blue Square campaign, to help combat antisemitism and hate in all forms. "CBS Saturday Morning" sits down with Kraft to learn more.