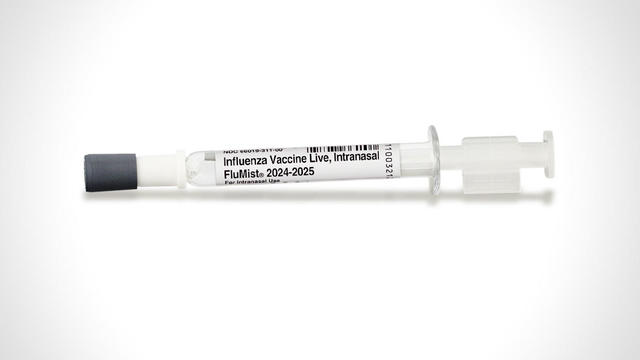
FDA approves first self-administered flu vaccine spray
AstraZeneca says the spray could be available "as soon as next flu season."
Watch CBS News

AstraZeneca says the spray could be available "as soon as next flu season."

Consumer Reports found elevated lead levels in 12 brands of cinnamon and spice blend products. Consumer Reports food safety reporter Lisa Gill joins CBS News to discuss the test and its findings.

Tech giant's latest AirPods will soon function as hearing aids for people with mild to moderate hearing loss.

Nearly 134,000 cases of multiple brands of apple juice are now being recalled because of of potential contamination.

Ultra-processed foods now make up over half of an average American adult's diet and two-thirds of an American child's.

Walmart has recalled nearly 10,000 cases of Great Value brand apple juice sold in stores across the U.S. due to potentially harmful levels of inorganic arsenic.

The new Food and Drug Administration-approved COVID-19 booster will address strains that are presently circulating in the U.S., according to Dr. William Schaffner, a professor in the Division of Infectious Diseases at Vanderbilt University Medical Center, who joins CBS News with more details.

The nasal spray "Neffy" is the first needle-free option for severe allergic reactions that the FDA has approved. The spray would be an alternative to EpiPens.

Drugmaker Lykos Therapeutics had asked the FDA to approve its MDMA capsules as part of a therapy regimen for treating PTSD. It says another study will take "several years."

Public health alert and multiple recalls issued after dangerous contaminant found in several brands of the spice.
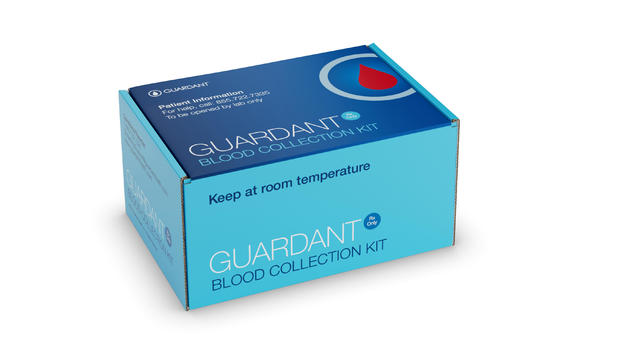
The Food and Drug Administration has approved a blood test intended to detect colorectal cancer, expanding options for screening for the potentially deadly disease.

The FDA has approved a new blood test for detecting colon cancer. The approval comes after a clinical study found the test could correctly detect colon cancer in 83% of participants who were not experiencing symptoms. CBS News chief medical correspondent Dr. Jon LaPook has the details.

The FDA has approved a product that screens for colorectal cancer using blood tests. Dr. William Schaffner, a professor of preventive medicine at Vanderbilt University Medical Center, joins CBS News with more details.

The anti-sunscreen movement is spreading misinformation online, and some younger adults are questioning sun safety.
The FDA found even some products that claimed to be "sterile" were contaminated.
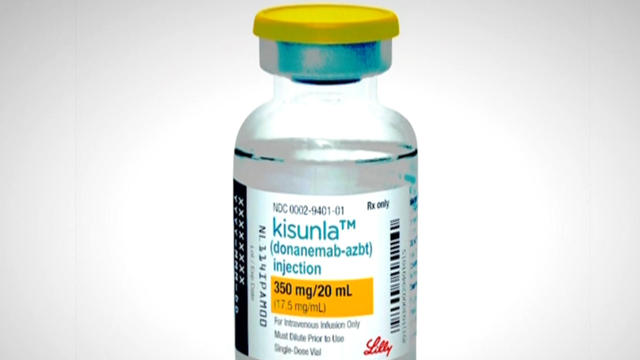
The FDA on Tuesday approved a new medication for people in the early stages of Alzheimer's disease. Dr. Jon LaPook explains what the drug is meant to do and explores its limitations.

Parent groups and anti-tobacco advocates blast FDA move to authorize vaping brand Njoy to market its products to the public.

Roughly 90% of Black women have used relaxers at some point in their lives to chemically straighten their hair. New reporting in The New York Times Magazine highlights the severe and often unknown health risks these products can pose. Linda Villarosa, contributing writer for The New York Times Magazine and the report's author, joins CBS News to unpack her findings.

The Supreme Court issued a unanimous ruling preserving access to the abortion pill mifepristone on Thursday. The justices ruled that the group of anti-abortion rights doctors who sued the FDA did not have the legal grounds to do so. CBS News legal contributor Jessica Levinson breaks down the decision.

The Supreme Court on Thursday preserved access to mifepristone, the nation's most prescribed abortion drug. Jan Crawford, CBS News chief legal correspondent, and Elizabeth Sepper, professor of law at the University of Texas at Austin, join "America Decides" to unpack the ruling.

It's the second week of June, which means the Supreme Court is expected to make some major decision as early as this Thursday. CBS News legal contributor Jessica Levinson joins to break down the most closely watched cases.

FDA advisers cited a variety of concerns with the trials submitted by Lykos Therapeutics as part of the application.

Private-label food supplier recalls 32-ounce pouches of Great Value Organic Black Chia Seeds because of potential contamination.

Interest in raw milk is rising in the U.S., fueled by both "wellness" and conservative influencers on social media — even though it can make people very sick.

Documents show a dental lab that made a device that was supposed to help patients with TMJ jaw disorder was never inspected by the FDA before a CBS News and KFF Health News investigation. Numerous patients have said the "anterior growth guidance appliance," or AGGA, damaged their mouths. The device's inventor, dentist Dr. Steve Galella, claimed it could cure TMJ jaw disorder and sleep apnea. The device and its inventor are under criminal investigation. They have denied wrongdoing. Anna Werner reports.

The official DHS statistics, which had not been previously reported, provide the most detailed look yet into who ICE has arrested during the Trump administration's crackdown.

"Today" show co-host Savannah Guthrie issued a plea for the public's help on Monday at what she called "an hour of desperation" in the search for her mother, Nancy.

U.S. Olympian Hunter Hess said "there is so much that is great about America, but there are always things that could be better," a day after President Trump lashed out at him.

Lindsey Vonn posted on Instagram a day after suffering a broken leg in a devastating crash at the Winter Olympics in Italy.

Google and Pepsi were among the best ads of the Big Game, while Coinbase and ai.com got failing grades, according to one ranking.

The Justice Department is moving to toss out its case against former Trump adviser Steve Bannon, who was jailed for declining to testify before the House Jan. 6 panel.

Ghislaine Maxwell's lawyer said she would be willing to cooperate with a House panel's probe if President Trump grants her clemency, and would testify that he is "innocent of any wrongdoing."

Taming runaway U.S. beef prices will require more than stepping up imports, economists said. Here's the key to cutting costs.

Here is a look at where the medal count stands for Team USA and other nations as the competition heats up in the 2026 Winter Olympics.

The Justice Department is moving to toss out its case against former Trump adviser Steve Bannon, who was jailed for declining to testify before the House Jan. 6 panel.

Researchers at two Spanish universities found that 84% of the contiguous U.S. states has shown signs of warming over the last 70 or so years, which is more than previously suggested.

ChatGPT will clearly distinguish between ads and answers to user prompts on the AI platform, according to OpenAI.

Taming runaway U.S. beef prices will require more than stepping up imports, economists said. Here's the key to cutting costs.

New items, such as a strawberry matcha loaf, represent the chain's latest effort to boost sales as part of its "Back to Starbucks" campaign.

ChatGPT will clearly distinguish between ads and answers to user prompts on the AI platform, according to OpenAI.

Taming runaway U.S. beef prices will require more than stepping up imports, economists said. Here's the key to cutting costs.

New items, such as a strawberry matcha loaf, represent the chain's latest effort to boost sales as part of its "Back to Starbucks" campaign.

Olympic medals have what's known as a "melt value." But they're worth far more financially than their mineral contents, an auction expert notes.

Eddie Bauer, a 106-year-old retailer, points to declining sales and "tariff certainty" as factors behind its latest move to seek bankruptcy protection.

U.S. Olympian Hunter Hess said "there is so much that is great about America, but there are always things that could be better," a day after President Trump lashed out at him.

The Justice Department is moving to toss out its case against former Trump adviser Steve Bannon, who was jailed for declining to testify before the House Jan. 6 panel.

Ghislaine Maxwell's lawyer said she would be willing to cooperate with a House panel's probe if President Trump grants her clemency, and would testify that he is "innocent of any wrongdoing."

The official DHS statistics, which had not been previously reported, provide the most detailed look yet into who ICE has arrested during the Trump administration's crackdown.

Rep. Tony Gonzales said the Dilley detention facility, which houses families and children, is "nicer than some elementary schools."

Ballad Health, the nation's largest state-sanctioned hospital monopoly, plans to rebuild Unicoi County Hospital in Tennessee on land that two climate modeling companies say is at risk of flooding.

Becca Valle, then 37, enrolled in a cutting-edge clinical trial after surgery removed an aggressive tumor from her brain.

More than three dozen cases of death cap mushroom poisonings have been reported in California since November, health officials said.

Here's what to know about TrumpRx, including how it works, who can use it, and how much money it can save.

The Trump administration launched its new TrumpRx direct-to-consumer prescription drug listing site late Thursday, part of a push to offer medication at steep discounts.

U.S. Olympian Hunter Hess said "there is so much that is great about America, but there are always things that could be better," a day after President Trump lashed out at him.

Here is a look at where the medal count stands for Team USA and other nations as the competition heats up at the 2026 Winter Olympics.

Taming runaway U.S. beef prices will require more than stepping up imports, economists said. Here's the key to cutting costs.

Team USA's mixed doubles curling gold medal match against Sweden is slated for Tuesday, Feb. 10.

Skier Tallulah Proulx, 17, was raised in the U.S., but she's making Olympic history as the Philippines' first female, and youngest athlete in any Winter Games.

Ingrid Fajaro, a staff writer at Billboard, joins CBS News 24/7 to discuss the cultural impact of Bad Bunny's halftime performance at Super Bowl LX.

Puerto Rican superstar Bad Bunny, born Benito Antonio Martínez Ocasio, is one of the most-streamed artists on the planet.

Ad Age editor-in-chief Jeanine Poggi joins "CBS Mornings" to break down which ads during Super Bowl LX stood out and if some missed the mark.

When self-proclaimed "Quad God" Ilia Malinin landed seven quad jumps in a single program last December, he boisterously ushered in a new era of skating with his daring routines.

The Seattle Seahawks beat the New England Patriots 29-13 in Super Bowl LX on Sunday. Bad Bunny's halftime show highlighted Puerto Rican culture and featured Ricky Martin and Lady Gaga, but drew criticism from President Trump. CBS News correspondent Nidia Cavazos has more.

ChatGPT will clearly distinguish between ads and answers to user prompts on the AI platform, according to OpenAI.

The FAA says it is collaborating with the FBI to detect, track and assess unauthorized drone activity at the Super Bowl.

From labor shortages to environmental impacts, farmers are looking to AI to help revolutionize the agriculture industry. One California startup, Farm-ng, is tapping into the power of AI and robotics to perform a wide range of tasks, including seeding, weeding and harvesting.

Gamers across the world can now recreate drone strikes in Ukraine from the comfort of their own home, with this newly released game.

CBS News business analyst Jill Schlesinger talks about how companies are using artificial intelligence, the discussion around the technology and how it's impacting the workforce.

After decades monitoring polar bears in Norway's far north, researchers say the animals have proven incredibly adaptable, but there are no guarantees for the future.

Dark matter doesn't absorb or give off light so scientists can't study it directly. But they can observe how its gravity warps and bends the star stuff around it.

"CBS Saturday Morning" learns more about Veronika, the clever cow who figured out multiple ways to scratch herself with a broom. It was the first time a cow was seen using a tool.
"Sunday Morning" looks back at historical events on this date.

The Dinosaur National Monument, which is located on the border between Colorado and Utah, was last excavated in 1924.

Savannah Guthrie released a new video on Monday, pleading for the public's help in finding her mother, Nancy. CBS News reporter Andres Gutierrez has the latest.

"Today" show co-host Savannah Guthrie posted a new video Monday pleading for the public's help in the search for her missing mother, Nancy. CBS News' Andres Gutierrez has more.

Police say one person is in custody after at least one person was shot at a Maryland high school on Monday. CBS affiliate WUSA reports.

"Today" show co-host Savannah Guthrie issued a plea for the public's help on Monday at what she called "an hour of desperation" in the search for her mother, Nancy.

At least one person has been shot at Thomas S. Wootton High School in Rockville, Maryland. The school is on lockdown and one person has been taken into custody.

The new crew will replace four station fliers who returned to Earth ahead of schedule last month due to a medical issue.

NASA's first crewed moon mission in more than 50 years has been delayed until March at the earliest. During a routine dress rehearsal of the launch, persistent liquid hydrogen leaks were discovered in the Artemis II rocket. CBS News space consultant Bill Harwood breaks it down.

NASA plans to test the planned leak repair with a second dress rehearsal fueling test later this month.

NASA delayed the Artemis II moon rocket launch after a hydrogen leak was found during a wet dress rehearsal, the agency announced Tuesday. CBS News senior space consultant Bill Harwood has the latest.

A NASA mission is underway to map the heliosphere, which is a huge protective bubble around the solar system that was created by the sun.

A look back at the esteemed personalities who've left us this year, who'd touched us with their innovation, creativity and humanity.

Does the evidence show a cover-up, or was Todd Kendhammer wrongfully convicted for the murder of his wife?

Christy Salters-Martin dominated in the boxing ring but faced her toughest challenger at home.

Family seeks answers in death of newlywed who disappeared in 2005 while on Mediterranean honeymoon cruise.

Meet the tattooed beauty charged in the death of Google executive Forrest Hayes.

According to a new Department of Homeland Security document obtained exclusively by CBS News, less than 14% of those arrested by ICE during President Trump's first year back in office had violent criminal records. CBS News immigration correspondent Camilo Montoya-Galvez has the details.

Savannah Guthrie released a new video on Monday, pleading for the public's help in finding her mother, Nancy. CBS News reporter Andres Gutierrez has the latest.

Congress is now permitted to view unredacted files related to the Justice Department's investigations into convicted sex offender Jeffrey Epstein. Jennifer Freeman, an attorney who represents several Epstein survivors, joins "The Takeout" to discuss.

Members of Congress went to the Justice Department on Monday to review unredacted files related to convicted sex offender Jeffrey Epstein. CBS News congressional correspondent Nicole Killion has the latest.

Bad Bunny's Super Bowl halftime show used several production elements to bring the artist's vision to life. Angela Watercutter, senior special projects editor for Wired, joined CBS News to discuss.