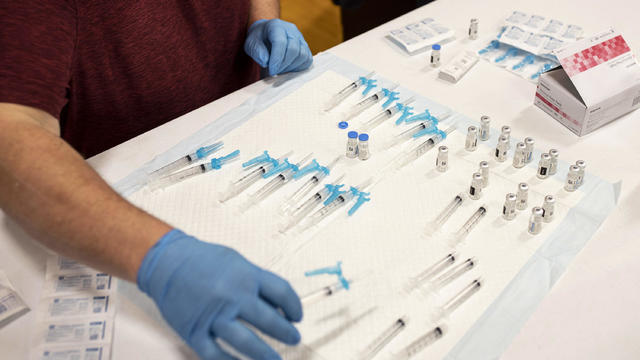
FDA authorizes Moderna and Johnson & Johnson booster shots
The CDC must also weigh in before additional doses can be administered nationwide.
Watch CBS News

The CDC must also weigh in before additional doses can be administered nationwide.
The FDA has authorized COVID-19 booster shots for some recipients of the Johnson and Johnson and Moderna vaccines. But as Nikki Battiste reports, the agency says eligible Americans can get any brand of booster, regardless of the shot they initially got. Then, Dr. Elizabeth Clayborne, an emergency physician and adjunct professor of emergency medicine at the University of Maryland School of Medicine, joins CBSN's Lana Zak with her analysis.

The FDA warns 75 brands of hand sanitizer may be making people sick, or even causing death if ingested. Kris Van Cleave reports.

After calling on churches to reopen this weekend, President Trump hit the golf course for a second straight day. This comes as some within his administration warn the coronavirus is not contained. Nikole Killion has the latest.

The Federal Drug Administration is moving fast on a potential coronavirus vaccine developed by drugmaker Moderna with the help of the National Institutes of Health. Dr. Jon LaPook has the details in CBS News' series "Racing to a Cure."
With Italy's economy under threat of collapse, the government says it's working on solutions to getting people back to work. In CBS News' latest installment of "Racing to a Cure," Chris Livesay shows us how they are using antibody tests to get things back to normal.

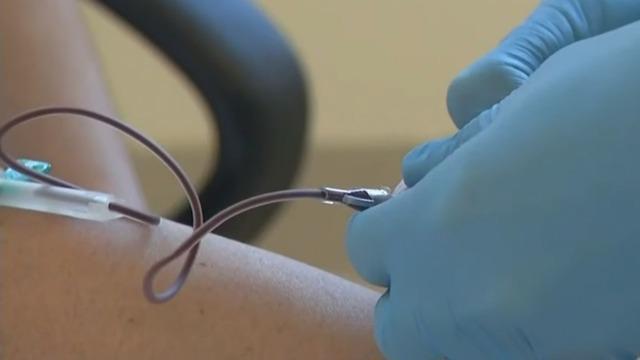
The FDA has authorized the first blood test for the coronavirus, which could identify people who have been exposed to the virus but do not show any symptoms. Dr. David Agus joins "CBS This Morning" to talk about the benefits of the new test and how it would help slow the coronavirus' spread.
The FDA has authorized a coronavirus test that can produce a positive result in five minutes, and a negative result in 13 minutes. To deal with the soaring number of cases, President Trump also said the FDA has approved a process to sterilize the N-95 masks that many hospitals are currently lacking. Dr. David Agus weighs in on both new developments during an appearance on "CBS This Morning."

Pharmacists across the country are raising the alarm about the over-prescription of drugs that may help treat the coronavirus. Recent data show chloroquine orders spiked 3,000% in March, and hydroxychloroquine orders rose 260%. The FDA has not approved these drugs for treatment of the virus, but doctors are allowed to prescribe them. Dr. Jon LaPook joins "CBS This Morning" to talk about the effects of over-prescribing these drugs.

CBS News is investigating misleading claims about coronavirus test kits. Catherine Herridge reports.

There may be a new hope in the desperate race to find a treatment for coronavirus and it couldn't come soon enough. President Trump says he is slashing red tape ordering the FDA to fast track the use of two drugs for sick patients. Ben Tracy has the latest.

Dr. Scott Gottlieb, the former FDA commissioner, said the CDC is likely to take a "cautious" approach in vaccinating kids ages 5 to 11, but it's "certainly reasonable" vaccination could start as soon as Thanksgiving.

U.S. launches strike on Baghdad airport; 98 year old swimmer proves she's still the best.

Senate still at impasse over impeachment trial; FDA issues ban on most flavored vaping products.
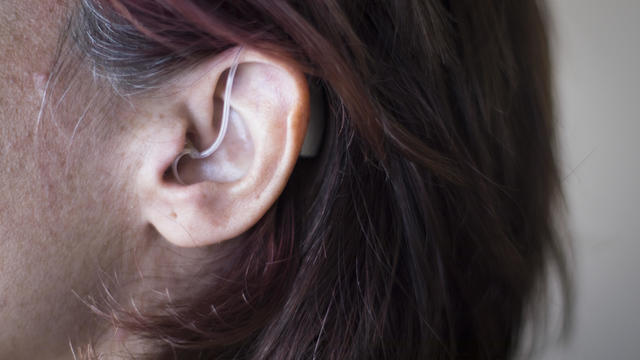
Americans with mild hearing loss may soon be able to buy hearing aids at their local drugstores.

Standoff between intel community and Congress; Md. boys' candle company finds success
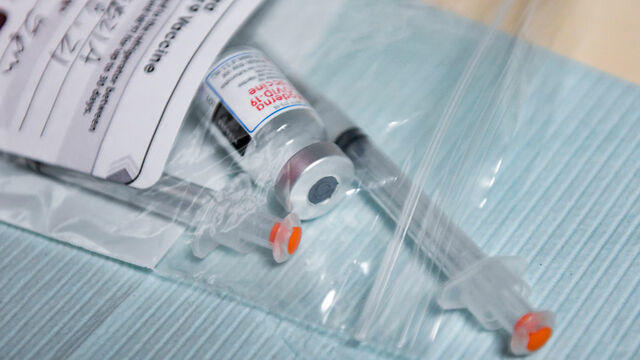
The Food and Drug Administration is reportedly close to approving "mixing and matching" COVID booster shots. CBS News' Skyler Henry reports that people could soon get a different shot than the one they originally received and Dr. Theodore Strange, chair of medicine at Staten Island University Hospital in New York City, joined CBSN to discuss what this means, especially for immunocompromised Americans.

The Food and Drug Administration is considering giving the green light to mixing and matching COVID-19 vaccine booster shots. Meanwhile, the death of former Secretary of State Colin Powell is focusing attention on so-called breakthrough COVID-19 cases among people with compromised immune systems. Dr. Jeremy Faust, an emergency physician at Brigham and Women's Hospital in Boston, joined CBSN to discuss.
The Food and Drug Administration is reportedly set to allow a mix-and-match approach for COVID-19 vaccine booster shots. According to The New York Times, the agency could announce its decision Wednesday, when it's expected to authorize the Moderna and Johnson & Johnson boosters. CBS News correspondent Laura Podesta joins CBSN AM to discuss.
The nation's top health agencies could approve boosters for the Moderna and Johnson & Johnson COVID vaccines this week. A CDC advisory panel is also meeting this week to discuss who should get the boosters. CBS News correspondent Laura Podesta joins CBSN AM to discuss.

The Biden administration is working with the private sector to try to ease ongoing supply chain issues. CBS News correspondent Carter Evans reports on the problem, and CBS News senior White House correspondent Weijia Jiang joins CBSN with a look at what the administration is doing on that plus, the latest FDA advisory meetings on potential COVID-19 booster shots from Moderna and Johnson & Johnson.
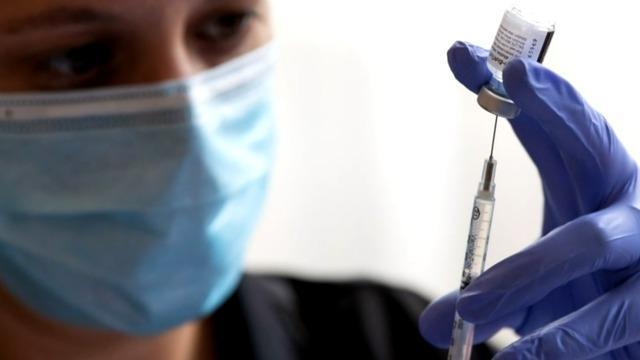
The FDA vaccine advisory panel is recommending booster shots for some Americans, and these recommendations will head to the FDA and CDC for further authorization. Dr. Ofer Levy, a voting member on that FDA vaccine panel, joins CBSN's Lana Zak for more on the story.
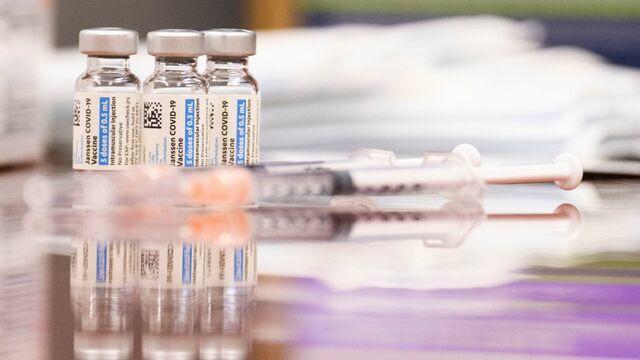
An FDA advisory panel has endorsed Johnson and Johnson's booster shot for some 15 million Americans. Mireya Villarreal has the details on the panel's decision. Then, Dr. Taison Bell, an assistant professor of medicine at the University of Virginia, joins CBSN's Lana Zak with his analysis.

An FDA panel recommended Johnson & Johnson's COVID-19 booster shot for adults 18 and older. The panel suggested getting the booster as early as two months after the original shot. Mireya Villarreal has more.
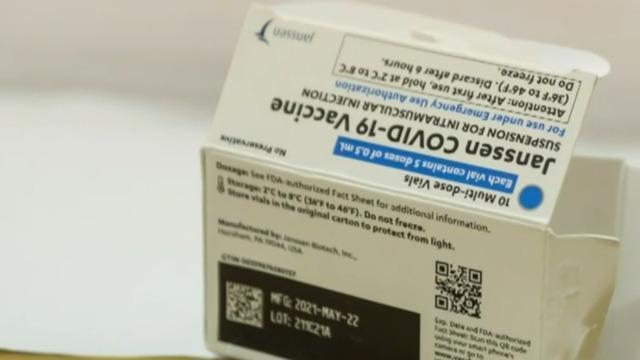
The FDA advisory panel unanimously voted to recommend the Johnson & Johnson booster shot on Friday. It comes a day after the same panel made the recommendation for a third Moderna shot. President Biden says full FDA and CDC approval for both boosters could come as early as next week. CBS News reporter Max Bayer and John Moore, a professor of microbiology and immunology at Weill Cornell Medical College, join CBSN's Tanya Rivero to discuss the FDA panel's vote.

The leaders of ICE, CBP and USCIS are testifying before the House Homeland Security Committee on Tuesday.

A Maryland mother is planning to self-deport after she was taken into ICE custody, causing her to miss her son's death.

A federal court in Georgia unsealed key records related to the FBI's seizure of 2020 election materials from Fulton County last month.

Kouri Richins allegedly poisoned her husband Eric by putting a fatal dose of fentanyl in his drink, leading to his sudden death in 2022.

Ben Ogden of Team USA won the silver medal in the cross-country sprint Tuesday at the Winter Olympics in Italy.
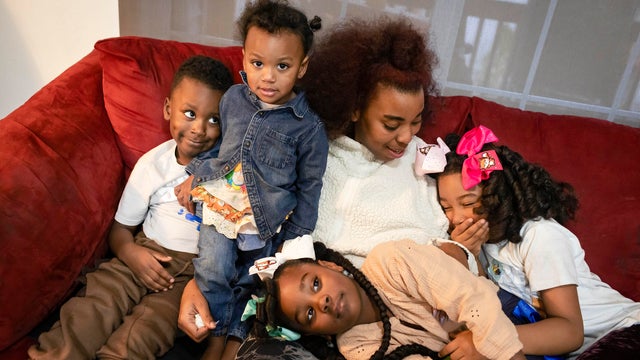
The Marshall Project found more than 70,000 cases referred to law enforcement over allegations of substance use during pregnancy — and that's a significant undercount.

"Today" show co-host Savannah Guthrie issued a plea for the public's help on Monday at what she called "an hour of desperation" in the search for her mother, Nancy.

CBS News medical contributor Dr. Céline Gounder said the results of the study on coffee drinkers having lower risk of dementia should be taken "with a massive grain of salt."

The Trump administration has filed lawsuits against 24 states in an effort to obtain their voter rolls.

Ketanji Brown Jackson told "CBS Mornings" that the justices "have learned how to adapt to being in an environment with people who have very strongly held but different views."

The Vatican Bank said the new indexes are "designed to serve as a reference for Catholic investments worldwide."

Kouri Richins allegedly poisoned her husband Eric by putting a fatal dose of fentanyl in his drink, leading to his sudden death in 2022.

A federal court in Georgia unsealed key records related to the FBI's seizure of 2020 election materials from Fulton County last month.

CBS News medical contributor Dr. Céline Gounder said the results of the study on coffee drinkers having lower risk of dementia should be taken "with a massive grain of salt."

The Vatican Bank said the new indexes are "designed to serve as a reference for Catholic investments worldwide."

Instagram's parent company Meta and Google's YouTube dispute claims that their platforms deliberately addict and harm children.

ChatGPT will clearly distinguish between ads and answers to user prompts on the AI platform, according to OpenAI.

Taming runaway U.S. beef prices will require more than stepping up imports, economists said. Here's the key to cutting costs.

New items, such as a strawberry matcha loaf, represent the chain's latest effort to boost sales as part of its "Back to Starbucks" campaign.

Ketanji Brown Jackson told "CBS Mornings" that the justices "have learned how to adapt to being in an environment with people who have very strongly held but different views."

A federal court in Georgia unsealed key records related to the FBI's seizure of 2020 election materials from Fulton County last month.

The Trump administration has filed lawsuits against 24 states in an effort to obtain their voter rolls.

A Canadian airline suspends flights to Cuba as U.S sanctions and Trump's tariff threats force Havana to warn carriers there's no way to refuel on the island.

Republican Sen. Susan Collins announced a widely expected reelection bid on Tuesday as focus turns to the Maine Senate race, which could be among the most consequential this cycle.

CBS News medical contributor Dr. Céline Gounder said the results of the study on coffee drinkers having lower risk of dementia should be taken "with a massive grain of salt."
The Marshall Project found more than 70,000 cases referred to law enforcement over allegations of substance use during pregnancy — and that's a significant undercount.

Experts say Affordable Care Act sign-up data won't be clear until people who were enrolled have paid — or not — their new, often much higher, premiums.

Ballad Health, the nation's largest state-sanctioned hospital monopoly, plans to rebuild Unicoi County Hospital in Tennessee on land that two climate modeling companies say is at risk of flooding.

Becca Valle, then 37, enrolled in a cutting-edge clinical trial after surgery removed an aggressive tumor from her brain.

A Canadian airline suspends flights to Cuba as U.S sanctions and Trump's tariff threats force Havana to warn carriers there's no way to refuel on the island.

Ben Ogden of Team USA won the silver medal in the cross-country sprint Tuesday at the Winter Olympics in Italy.

King Charles II says the royal family will support U.K. police as they look into a report that the monarch's brother Andrew shared secret info with Epstein.

The International Olympic Committee has barred a Ukrainian skeleton racer from wearing a helmet with images of fellow athletes killed in Russia's invasion.

Marius Borg Hoiby, Crown Princess Mette-Marit's 29-year-old son, is on trial facing 38 charges, including raping four women and assaults against ex-girlfriends.

Chappell Roan says she's left her talent agency after its CEO, Casey Wasserman, was named in files related to late convicted sex offender Jeffrey Epstein.

Just 30 seconds of highly coveted commercial airtime during the Super Bowl costs as much as $10 million, according to CBS News MoneyWatch. Bill Pearce, marketing faculty member at The University of California, Berkeley, joins to discuss some of the ads from Super Bowl LX.

Bad Bunny's historic Super Bowl halftime show included superstar surprise guests and a message of unity and cultural celebration. While many praised the performance, President Trump took to social media to criticize the show. CBS News political director Fin Gómez joins with analysis.

The Super Bowl is a football game, an entertainment spectacle, a global billboard and a crucible of American political discord. CBS News chief Washington correspondent Major Garrett explains.

Catherine O'Hara, known for her roles in "Home Alone," "Schitt's Creek" and "Beetlejuice," died on Jan. 30 at the age of 71.
The demands of the artificial intelligence boom may be causing shortages in other sectors that help boost the U.S. economy. Shira Ovide, a technology reporter for The Washington Post, joins CBS News with more.

Opening statements began in a landmark trial against Google and Meta on the apparent harms of social media platforms. CBS News' Jo Ling Kent reports.

From labor shortages to environmental impacts, farmers are looking to AI to help revolutionize the agriculture industry. One California startup, Farm-ng, is tapping into the power of AI and robotics to perform a wide range of tasks, including seeding, weeding and harvesting.

Instagram's parent company Meta and Google's YouTube dispute claims that their platforms deliberately addict and harm children.

Opening statements began Monday in Los Angeles in a landmark trial over alleged social media addiction in children. CBS News correspondent Carter Evans has the details.

After decades monitoring polar bears in Norway's far north, researchers say the animals have proven incredibly adaptable, but there are no guarantees for the future.

Dark matter doesn't absorb or give off light so scientists can't study it directly. But they can observe how its gravity warps and bends the star stuff around it.

"CBS Saturday Morning" learns more about Veronika, the clever cow who figured out multiple ways to scratch herself with a broom. It was the first time a cow was seen using a tool.
"Sunday Morning" looks back at historical events on this date.

The Dinosaur National Monument, which is located on the border between Colorado and Utah, was last excavated in 1924.

Kouri Richins allegedly poisoned her husband Eric by putting a fatal dose of fentanyl in his drink, leading to his sudden death in 2022.

The FBI is now offering a $50,000 reward in the search for Nancy Guthrie, who was reported missing on Feb. 1. CBS News' Andres Gutierrez and Anna Schecter have the latest.

Ghislaine Maxwell invoked the Fifth Amendment during her congressional testimony on the Jeffrey Epstein investigation. This comes as the world reacts to the latest batch of documents released by the Justice Department. CBS News' Katrina Kaufman and Holly Williams have more.

The search for Savannah Guthrie's mom, Nancy, continues 10 days after she went missing in Arizona. CBS News' Jonathan Vigliotti has the latest news.

Opening statements began in a landmark trial against Google and Meta on the apparent harms of social media platforms. CBS News' Jo Ling Kent reports.

The new crew will replace four station fliers who returned to Earth ahead of schedule last month due to a medical issue.

NASA's first crewed moon mission in more than 50 years has been delayed until March at the earliest. During a routine dress rehearsal of the launch, persistent liquid hydrogen leaks were discovered in the Artemis II rocket. CBS News space consultant Bill Harwood breaks it down.

NASA plans to test the planned leak repair with a second dress rehearsal fueling test later this month.

NASA delayed the Artemis II moon rocket launch after a hydrogen leak was found during a wet dress rehearsal, the agency announced Tuesday. CBS News senior space consultant Bill Harwood has the latest.

A NASA mission is underway to map the heliosphere, which is a huge protective bubble around the solar system that was created by the sun.

A look back at the esteemed personalities who've left us this year, who'd touched us with their innovation, creativity and humanity.

Does the evidence show a cover-up, or was Todd Kendhammer wrongfully convicted for the murder of his wife?

Christy Salters-Martin dominated in the boxing ring but faced her toughest challenger at home.

Family seeks answers in death of newlywed who disappeared in 2005 while on Mediterranean honeymoon cruise.

Meet the tattooed beauty charged in the death of Google executive Forrest Hayes.

President Trump said that he "made a mistake" by not nominating Kevin Warsh for Federal Reserve chair during his first term. Kristin Myers, the ETF editor-in-chief for AssetTV, joins with more.

The White House said it was not inviting any Democrats to the National Governors Association's annual meeting next week with the president, a traditionally bipartisan event. CBS News' Natalie Brand has more.

Rep. Bennie Thompson, the top democrat on the House Homeland Security Committee, questioned the leaders of three immigration agencies about body cameras and training time for officers, and asked whether they have been involved in planning for guarding voting precincts. His last question comes after President Trump called on Republicans to "nationalize" elections.

GOP Rep. Michael McCaul of Texas questioned acting Director Todd Lyons about the situation in Minneapolis after the fatal shootings of Renee Good and Alex Pretti. Lyons said he's "seen a deescalation" in the city after border czar Tom Homan was sent to manage the immigration operation.

The Democratic Congressional Campaign Committee announced it's targeting five additional seats in Republican-held districts ahead of the 2026 midterm elections. Meanwhile, President Trump said that Republicans "should win in a landslide." CBS News' Aaron Navarro has more.