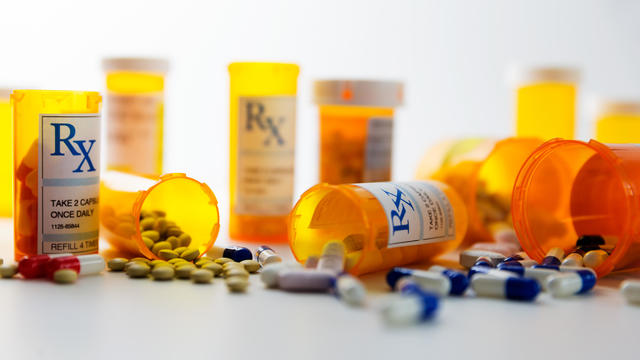
ADHD medication recalled because bottles may contain a different drug
Azurity Pharmaceuticals is recalling Zanzedi after a pharmacist found a pill with sedative effects in a package of the stimulant drug.
Watch CBS News

Azurity Pharmaceuticals is recalling Zanzedi after a pharmacist found a pill with sedative effects in a package of the stimulant drug.

The FDA took the extraordinary step to approve the over-the-counter sale of Narcan, a drug that can quickly reverse the effects of opioid overdoses. CBS News medical contributor and editor-at-large for public health at Kaiser Family Foundation, Dr. Celine Gounder joins "CBS Mornings" to discuss the significance of the approval and how someone should administer the drug.

FDA testing found "extremely high levels of lead contamination" in the cinnamon applesauce pouches, which have been recalled.

Quaker Oats expands prior recall to include more granola bars, cereals and a snack mix possibly tainted with bacteria.

Ozempic and other GLP-1 RA drugs, including Wegovy, Mounjaro and Zepbound, are being scrutinized.

Five flavors of Benny T's Vesta dry hot sauce are being recalled for having labels that fail to disclose allergens in the products.
For the first time, the FDA is allowing a Florida health program to import some prescription drugs in bulk from Canada. Stacie Dusetzina, professor of health policy at Vanderbilt University, joins CBS News to explain why the state is facing pushback on the plan.

Among the supermarket foods tested, Annie's Organic Cheesy Ravioli, Del Monte sliced peaches and Chicken of the Sea pink salmon, had the most phthalates per nanogram.

Kratom is commonly marketed as a health wonder, but the FDA warns of "serious adverse effects." It has even been blamed for several deaths. Mark Strassmann reports.
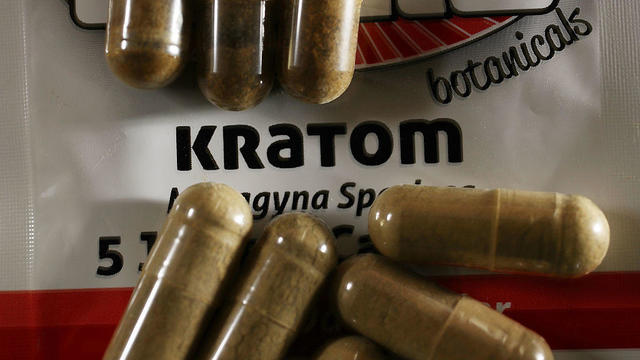
Kratom is commonly marketed as a wellness wonder, but the FDA warns not to use it due to the "risk of serious adverse effects."
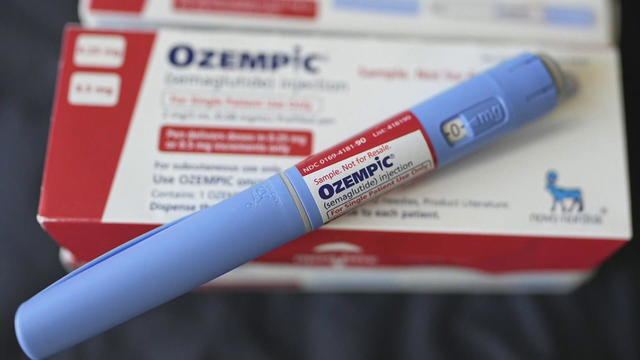
The FDA and drugmaker Novo Nordisk are working to identify thousands of counterfeit Ozempic drugs seized in an ongoing investigation.

Thousands of medical devices are sold, and even implanted, with no safety tests.

The HeartMate 3 is considered the safest mechanical heart pump of its kind, but a federal database contains more than 4,500 reports in which it may have caused or contributed to a patient's death.

There is growing concern over a lead poisoning outbreak tied to pouches of cinnamon applesauce for children, which one FDA official says could have been "intentional." CBS News' Meg Oliver reports.

Coca-Cola's recall impacts 12-pack cans of soda distributed in Alabama, Mississippi and Florida, the FDA said in a filing.

The Supreme Court took on two new cases Wednesday: One on the abortion pill, and the other relating to Jan. 6 and former President Donald Trump. Jan Crawford and Jessica Levinson explore the issues at stake in each case.

Federal officials issued their first guidelines on preparing morel mushrooms after a deadly food poisoning outbreak in Montana, noting the toxins in the delicacy aren't fully understood.

The FDA is set to decide Friday on a gene editing technique for patients with sickle cell disease. It would be the first use of CRISPR in the United States to treat a disease. CBS News' chief medical correspondent Dr. Jon LaPook has more.

The Biden administration is holding off on banning menthol cigarettes -- even though health experts say doing so would save lives. Dr. Enid Neptune, scientific adviser for the American Lung Association, joins CBS News to discuss.

Consuming some caffeine is typically harmless for adults, but having too much can be dangerous. Here's what to know about potential health effects.

The FDA and the manufacturer were alerted to Profemur titanium hips breaking inside U.S. patients as of 2005. It took 15 years to recall the devices. Many fractures could have been avoided.

Cantaloupes contaminated with the bacteria have been linked to more than 100 illnesses and two deaths, health officials said.

CDC investigating fruit from HMC Farms after one person died and 10 are hospitalized following a listeria outbreak.

The illegal Neptune's Fix brand supplements claim to contain tianeptine, known as "gas station heroin."
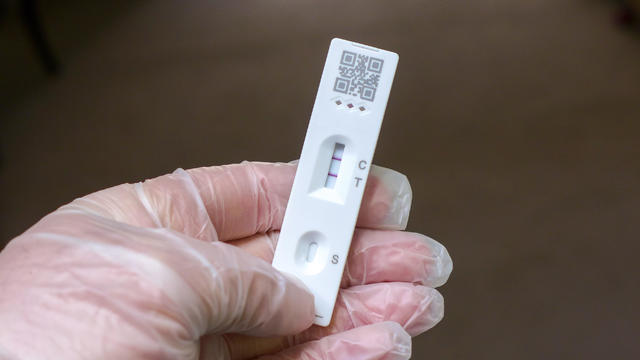
The FDA has lengthened the shelf lives of some COVID-19 tests that still deliver accurate results past their printed expiration dates.

The official DHS statistics, which had not been previously reported, provide the most detailed look yet into who ICE has arrested during the Trump administration's crackdown.

"Today" show co-host Savannah Guthrie issued a plea for the public's help on Monday at what she called "an hour of desperation" in the search for her mother, Nancy.

U.S. Olympian Hunter Hess said "there is so much that is great about America, but there are always things that could be better," a day after President Trump lashed out at him.

The U.S. military struck its 39th alleged drug-carrying boat on Monday, killing two people and leaving one survivor who is now the focus of a search-and-rescue effort.

The Justice Department is moving to toss out its case against former Trump adviser Steve Bannon, who was jailed for declining to testify before the House Jan. 6 panel.

Google and Pepsi were among the best ads of the Big Game, while Coinbase and ai.com got failing grades, according to one ranking.

Taming runaway U.S. beef prices will require more than stepping up imports, economists said. Here's the key to cutting costs.

Here is a look at where the medal count stands for Team USA and other nations as the competition heats up in the 2026 Winter Olympics.

Catherine O'Hara, known for her roles in "Home Alone," "Schitt's Creek" and "Beetlejuice," died on Jan. 30 at the age of 71.

Instagram's parent company Meta and Google's YouTube dispute claims that their platforms deliberately addict and harm children.

A federal judge has blocked a California law from going into effect that would ban federal immigration agents from covering their faces but they will still be required to wear clear identification showing their agency and badge number.

Catherine O'Hara, known for her roles in "Home Alone," "Schitt's Creek" and "Beetlejuice," died on Jan. 30 at the age of 71.

The Justice Department is moving to toss out its case against former Trump adviser Steve Bannon, who was jailed for declining to testify before the House Jan. 6 panel.

Researchers at two Spanish universities found that 84% of the contiguous U.S. states has shown signs of warming over the last 70 or so years, which is more than previously suggested.

Instagram's parent company Meta and Google's YouTube dispute claims that their platforms deliberately addict and harm children.

ChatGPT will clearly distinguish between ads and answers to user prompts on the AI platform, according to OpenAI.

Taming runaway U.S. beef prices will require more than stepping up imports, economists said. Here's the key to cutting costs.

New items, such as a strawberry matcha loaf, represent the chain's latest effort to boost sales as part of its "Back to Starbucks" campaign.

Olympic medals have what's known as a "melt value." But they're worth far more financially than their mineral contents, an auction expert notes.

The U.S. military struck its 39th alleged drug-carrying boat on Monday, killing two people and leaving one survivor who is now the focus of a search-and-rescue effort.

A federal judge has blocked a California law from going into effect that would ban federal immigration agents from covering their faces but they will still be required to wear clear identification showing their agency and badge number.

U.S. Olympian Hunter Hess said "there is so much that is great about America, but there are always things that could be better," a day after President Trump lashed out at him.

The Justice Department is moving to toss out its case against former Trump adviser Steve Bannon, who was jailed for declining to testify before the House Jan. 6 panel.

Ghislaine Maxwell's lawyer said she would be willing to cooperate with a House panel's probe if President Trump grants her clemency, and would testify that he is "innocent of any wrongdoing."

Ballad Health, the nation's largest state-sanctioned hospital monopoly, plans to rebuild Unicoi County Hospital in Tennessee on land that two climate modeling companies say is at risk of flooding.

Becca Valle, then 37, enrolled in a cutting-edge clinical trial after surgery removed an aggressive tumor from her brain.

More than three dozen cases of death cap mushroom poisonings have been reported in California since November, health officials said.

Here's what to know about TrumpRx, including how it works, who can use it, and how much money it can save.

The Trump administration launched its new TrumpRx direct-to-consumer prescription drug listing site late Thursday, part of a push to offer medication at steep discounts.

U.S. Olympian Hunter Hess said "there is so much that is great about America, but there are always things that could be better," a day after President Trump lashed out at him.

Here is a look at where the medal count stands for Team USA and other nations as the competition heats up at the 2026 Winter Olympics.

Taming runaway U.S. beef prices will require more than stepping up imports, economists said. Here's the key to cutting costs.

Team USA's mixed doubles curling gold medal match against Sweden is slated for Tuesday, Feb. 10.

Skier Tallulah Proulx, 17, was raised in the U.S., but she's making Olympic history as the Philippines' first female, and youngest athlete in any Winter Games.

Just 30 seconds of highly coveted commercial airtime during the Super Bowl costs as much as $10 million, according to CBS News MoneyWatch. Bill Pearce, marketing faculty member at The University of California, Berkeley, joins to discuss some of the ads from Super Bowl LX.

Bad Bunny's historic Super Bowl halftime show included superstar surprise guests and a message of unity and cultural celebration. While many praised the performance, President Trump took to social media to criticize the show. CBS News political director Fin Gómez joins with analysis.

The Super Bowl is a football game, an entertainment spectacle, a global billboard and a crucible of American political discord. CBS News chief Washington correspondent Major Garrett explains.

Catherine O'Hara, known for her roles in "Home Alone," "Schitt's Creek" and "Beetlejuice," died on Jan. 30 at the age of 71.

Ingrid Fajaro, a staff writer at Billboard, joins CBS News 24/7 to discuss the cultural impact of Bad Bunny's halftime performance at Super Bowl LX.

Instagram's parent company Meta and Google's YouTube dispute claims that their platforms deliberately addict and harm children.

Opening statements began Monday in Los Angeles in a landmark trial over alleged social media addiction in children. CBS News correspondent Carter Evans has the details.

From labor shortages to environmental impacts, farmers are looking to AI to help revolutionize the agriculture industry. One California startup, Farm-ng, is tapping into the power of AI and robotics to perform a wide range of tasks, including seeding, weeding and harvesting.

ChatGPT will clearly distinguish between ads and answers to user prompts on the AI platform, according to OpenAI.

The FAA says it is collaborating with the FBI to detect, track and assess unauthorized drone activity at the Super Bowl.

After decades monitoring polar bears in Norway's far north, researchers say the animals have proven incredibly adaptable, but there are no guarantees for the future.

Dark matter doesn't absorb or give off light so scientists can't study it directly. But they can observe how its gravity warps and bends the star stuff around it.

"CBS Saturday Morning" learns more about Veronika, the clever cow who figured out multiple ways to scratch herself with a broom. It was the first time a cow was seen using a tool.
"Sunday Morning" looks back at historical events on this date.

The Dinosaur National Monument, which is located on the border between Colorado and Utah, was last excavated in 1924.

A second ransom deadline passed Monday for Nancy Guthrie, the mother of "Today" show co-host Nancy Guthrie. CBS News correspondent Jonathan Vigliotti reports on the search and former FBI special agent Jeff Harp joins to discuss the situation.

The children of Nancy Guthrie, including "Today" show co-anchor Savannah Guthrie, are praying for signs of life more than a week after their mother disappeared. CBS News reporter Andres Gutierrez has more from Tucson, Arizona.

Savannah Guthrie released a new video on Monday, pleading for the public's help in finding her mother, Nancy. CBS News reporter Andres Gutierrez has the latest.

"Today" show co-host Savannah Guthrie posted a new video Monday pleading for the public's help in the search for her missing mother, Nancy. CBS News' Andres Gutierrez has more.

Police say one person is in custody after at least one person was shot at a Maryland high school on Monday. CBS affiliate WUSA reports.

The new crew will replace four station fliers who returned to Earth ahead of schedule last month due to a medical issue.

NASA's first crewed moon mission in more than 50 years has been delayed until March at the earliest. During a routine dress rehearsal of the launch, persistent liquid hydrogen leaks were discovered in the Artemis II rocket. CBS News space consultant Bill Harwood breaks it down.

NASA plans to test the planned leak repair with a second dress rehearsal fueling test later this month.

NASA delayed the Artemis II moon rocket launch after a hydrogen leak was found during a wet dress rehearsal, the agency announced Tuesday. CBS News senior space consultant Bill Harwood has the latest.

A NASA mission is underway to map the heliosphere, which is a huge protective bubble around the solar system that was created by the sun.

A look back at the esteemed personalities who've left us this year, who'd touched us with their innovation, creativity and humanity.

Does the evidence show a cover-up, or was Todd Kendhammer wrongfully convicted for the murder of his wife?

Christy Salters-Martin dominated in the boxing ring but faced her toughest challenger at home.

Family seeks answers in death of newlywed who disappeared in 2005 while on Mediterranean honeymoon cruise.

Meet the tattooed beauty charged in the death of Google executive Forrest Hayes.

"Today" show co-host Savannah Guthrie issued a plea for the public's help at what she called "an hour of desperation." As Jonathan Vigliotti reports, the search for her missing mom, Nancy Guthrie, has entered its second week with few new leads and no new suspects.

Just 30 seconds of highly coveted commercial airtime during the Super Bowl costs as much as $10 million, according to CBS News MoneyWatch. Bill Pearce, marketing faculty member at The University of California, Berkeley, joins to discuss some of the ads from Super Bowl LX.

Nick Emmanwori just won it all in his rookie season as a safety with the Seattle Seahawks, and his mom was somewhere in that cheering crowd of 70,000. But Justina Emmanwori is not your typical football mom. Tony Dokoupil has more on her viral interview.

Bad Bunny's historic Super Bowl halftime show included superstar surprise guests and a message of unity and cultural celebration. While many praised the performance, President Trump took to social media to criticize the show. CBS News political director Fin Gómez joins with analysis.

Ghislaine Maxwell, a longtime associate of convicted sex offender Jeffrey Epstein who herself was convicted of sex trafficking in 2021, invoked her Fifth Amendment right against self-incrimination and refused to answer questions during a virtual appearance before the House Oversight and Government Reform Committee. Scott MacFarlane reports.