Study: Pfizer vaccine reduces transmission
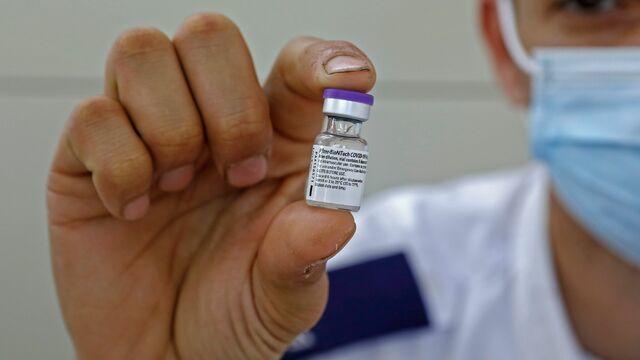
A new U.K. study found the Pfizer coronavirus vaccine reduced transmission after just one dose. Dr. Bob Lahita joins CBSN to discuss the findings, as well as the possible need for booster shots against new variants, a new CBS News poll on attitudes about vaccination, and the process of reopening schools amid the pandemic.