Thousands of COVID tests recalled over bacteria risk, FDA warns
More than a half million home COVID-19 tests from Roche and SD Biosensor should be thrown out immediately, the Food and Drug Administration is warning, citing "significant concerns" over bacteria that could infect users of the tests.
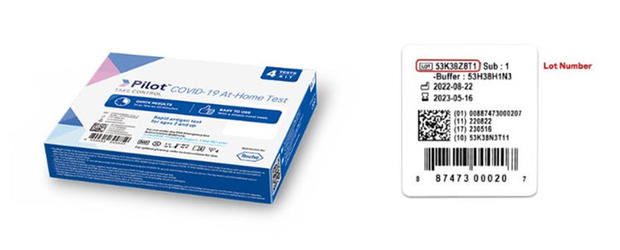
The FDA says the recalled "Pilot COVID-19 At-Home Tests" can be identified by lot numbers listed on this page. 500,000 were distributed to CVS and 16,000 to Amazon.
None of the potentially contaminated kits were distributed through the federal government's testing programs like COVID.gov/tests, the FDA says.
"SD Biosensor Inc., the manufacturer of the Pilot COVID-19 At-Home Test, informed Roche that this issue was identified during routine quality assurance testing. Potentially harmful bacteria were found in the liquid buffer solution," Roche said in a statement.
The FDA says bacteria discovered in liquid tubes that come with the test kits include Enterococcus, Enterobacter, Klebsiella and Serratia, which can lead to potentially dangerous infections, especially in people with compromised immune systems.
So far, the FDA says it has not received any reports of injuries or deaths linked to contaminated tests.
The agency also warns that people who have already used the tests may have gotten false positive or false negative results.
Roche and SD Biosensor said they have worked with distributors and retailers to quarantine lots of the tests while their investigation continues, and are cooperating with the FDA.
Evie Baik, a spokesperson for SD Biosensor, said in a statement that their probe has identified raw materials from one of their suppliers as the likely culprit.
The company is ramping up their quality control efforts, Baik said, in hopes of rooting out further contaminated batches before distribution. SD Biosensor also cut off the supplier behind the contamination.
"To date, no such illness has been reported and to date no impact on performance has been confirmed," Baik said.