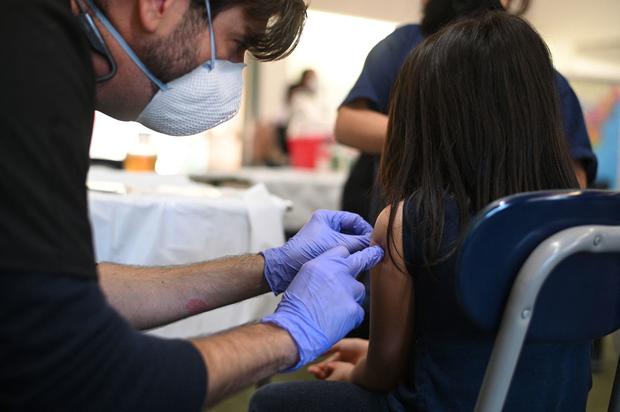
CDC clears Pfizer's COVID-19 vaccine boosters for children as young as five
A panel of the Centers for Disease Control and Prevention's outside vaccine advisers voted nearly unanimously on Thursday to recommend a third dose of Pfizer's COVID-19 vaccine for children as young as five years old, as early as five months after the second shot.
CDC Director Dr. Rochelle Walensky signed off on the recommendations Thursday evening, clearing the final hurdle before the additional shots can be rolled out.
"With over 18 million doses administered in this age group, we know that these vaccines are safe, and we must continue to increase the number of children who are protected. I encourage parents to keep their children up to date with CDC's COVID-19 vaccine recommendations," Walensky said in a statement.
Walensky's decision to clear the additional shots comes as the country is facing a renewed surge of COVID-19 cases and hospitalizations driven by different Omicron subvariants than those that fueled the winter surge.
A growing share of the country is now seeing levels of disease high enough to warrant masking by all Americans indoors, according to the CDC's data. Hospitalizations have surged among the oldest Americans, beyond peaks seen during the Delta variant wave last year.
The CDC also said Thursday it would step up its recommendation that adults ages 50 and older, as well as Americans who are immunocompromised, receive a second booster.
The groups had previously been allowed to receive the extra dose, but the agency had said some — like those who had survived an infection recently — could wait. Receiving the second dose had previously not been required to be counted as "up to date."
"While older Americans have the highest coverage of any age group of first booster doses, most older Americans received their last dose (either their primary series or their first booster dose) many months ago, leaving many who are vulnerable without the protection they may need to prevent severe disease, hospitalization, and death," the agency said.
Walensky's moves comes after a day-long meeting of the agency's advisers weighing the potential benefits a third dose could offer an age group that has largely survived COVID-19 infections with lagging rates of vaccination.
Less than a third of children ages five to 11 years old are fully vaccinated with two shots of Pfizer and BioNTech's COVID-19 vaccine and will become eligible for the booster.
The agency's surveys suggest vaccine hesitancy has climbed among children, but remains a minority. Approximately 39% of parents of children in the age group say they "probably or definitely" will not ever get their kids vaccinated.
CDC figures presented to the advisers suggest that around 77% of children in the age group have antibodies from at least one prior infection at some point during the pandemic.
However, those antibodies from prior infections did little to fend off a large wave of COVID-19 cases and hospitalizations in an age group where the disease now ranks as a leading cause of death.
By contrast, the agency officials estimated that some 1,000 hospitalizations were prevented by vaccination among the 7.5 million children who have received two shots.
How long that protection against hospitalization will last for the youngest children is unclear, officials told the group.
Findings from the agency's ongoing studies presented to the committee suggest vaccine effectiveness against infection declined quickly in children following two doses during the Omicron wave. Vaccine effectiveness against hospitalization also fell among unboosted adolescents 12 to 15 years old, but there was not enough data to confirm whether protection against hospitalization is waning in unboosted children ages 5 to 11 years old.
"It wouldn't make sense that five to 11 year olds are the only group among the age eligible for whom a third dose isn't necessary to achieve a more durable and effective immune response," Dr. Matthew Daley, head of the committee's work group on COVID-19 vaccines, said ahead of the vote.
Like in older age groups, data presented to the panel from 401 children in Pfizer and BioNTech's vaccine trials suggests the third dose raised no new safety risks.
The company also found the third shot offered a significant increase in antibodies, regardless of whether the child had a previous infection. However, that was measured in children who received the third dose as late as nine months after their second shot.
"The expectation would be that all the antibody responses might be a little bit lower, but they still should be providing additional protection, much as you've seen in other populations," Dr. William Gruber, head of Pfizer's vaccine research, told the committee.
Reactions to the third dose were largely mild or moderate, another Pfizer scientist told the panel. Only a handful of side effects – like headache, chills, or muscle pain – were reported slightly more frequently in recipients of the third shot.
"Data do not suggest potential safety concerns regarding a Pfizer-BioNTech COVID-19 vaccine booster for children five to 11 years of age, beyond those previously identified in older age groups," Dr. Keipp Talbot, head of the panel's vaccine safety work group, said.