Same COVID Test, Different Results: FDA Looking Into State Lab 'Test Result Concerns'
SACRAMENTO (CBS13) — The FDA says it's looking into "test result concerns" related to the PerkinElmer COVID test at the center of the state's $1.7 billion dollar lab contract.
CBS13 has learned that labs across the state are using the same PerkinElmer COVID test but are giving people different results for the same measure of virus. Records also reveal that the state lab first reported those results as "Negative," then "Inconclusive" and "Presumptive Positive" -- while other labs still call them "Positive" based on the FDA authorization.
Same COVID test, the same measure of the virus, several different results. So, who is right? Are people getting inaccurate results? CBS13 is getting answers.
THE IMPACT OF A POSITIVE TEST
Like many, Tim Connors decided to get tested for COVID ahead of planned travel for work. He didn't have any symptoms, but he'd heard that the Biden administration was considering requiring a negative test for domestic travel.
When Tim decided to get tested, he never expected the positive result that upended his life.
"I was shocked, to say the least," Tim said. "I had no symptoms, I had no reason to be tested in the first place other than I was planning to travel the following week."
Tim got his positive result on Super Bowl Sunday from the state's new PerkinElmer COVID testing lab. He tested negative at a local hospital the next day but he says the damage was done.
"Once you get that label that you're positive, there is absolutely nothing you can do to change it," he said.
CONTINUING COVERAGE: CBS13 Investigates Problems at California's COVID Testing Lab
Tim had to cancel his business trip and says the impact of his test was exponential.
He says his coworkers and his wife, a schoolteacher, were also forced to quarantine for 14 days due to their respective company and school district policies for exposure.
"Not only did it impact my life, but it impacted my family's life, my coworkers' lives, even my neighbors," Tim said.
Tim got subsequent negative results from two other labs. None of his close contacts ever tested positive. Now, he wonders if he got inaccurate results from the state's COVID testing lab.
Like most whose tests are processed at the state lab, Tim received a template form letter notifying him of his result. However, he's been unable to get the lab, or health officials, to provide him with a detailed lab report.
INVESTIGATING THE STATE LAB
Tim reached out to CBS13 after seeing our reports on the state's new COVID testing lab. The $25 million lab and $1.7 billion contract with PerkinElmer was supposed to revolutionize COVID testing in California.
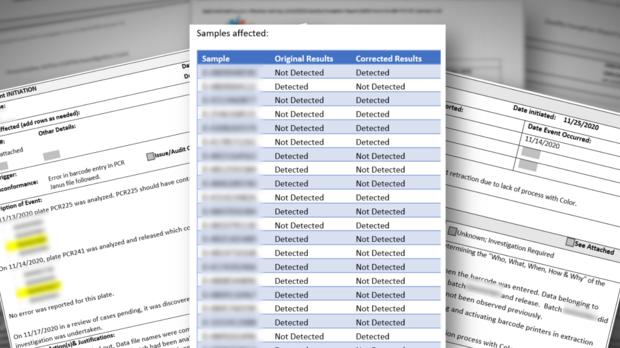
In November, CBS13 discovered a high number of inconclusive test results coming out of the state lab. State data later confirmed the lab released more than 36,000 inconclusive results during the 3-month peak of the pandemic, along with more than 13,000 invalid results, and 205 lost samples.
In all, more than 50,000 results did not indicate if the patient actually had COVID.
ALSO READ: Asleep At the Lab: Whistleblower Allegations From Inside CA Billion-Dollar COVID Lab
After seeing the initial reports, whistleblowers reached out to CBS13 to reveal what they say is going on inside the state lab.
We heard from more than a dozen current and former employees who provided evidence to support allegations ranging from unlicensed lab techs watching videos and literally sleeping while processing nasal swabs for testing, to incidents of contamination, swapped samples and wrong results.
Evidence also indicated that staff were processing patient samples before completing required training or getting signed-off for competency.
ALSO READ: Questionable COVID Results – More Concerning Whistleblower Allegations From State COVID Lab
Following the February whistleblower reports, the state announced an investigation into the allegations and acknowledged that inspectors previously found "significant deficiencies" at the lab during an unrelated routine inspection in December.
According to this document from the state, it appears the lab was facing an "immediate jeopardy" designation which denotes "the most severe and egregious threat to the health and safety." The lab had until March to address the deficiencies.
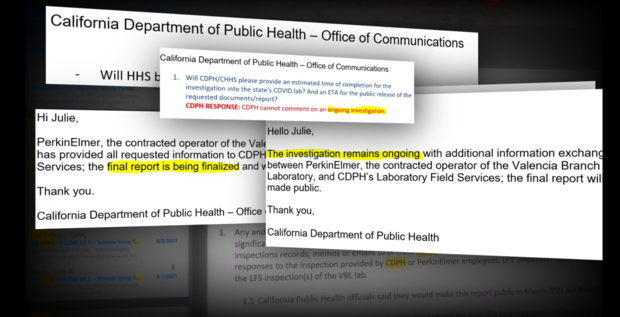
State regulators vowed to make the full inspection reports public in mid March. However, this week, the last full week of March, CDPH said the reports are not complete. CBS13 has learned inspectors were at the lab again late last week.
CHANGING THE CUTOFF FOR "POSITIVE" RESULTS
CBS13's investigation also revealed the lab changed the cutoff for "Positive" results, calling low measures of the virus "Inconclusive" that the FDA says should be reported as "Positive" according to PerkinElmer's own FDA authorized COVID test and data.
We've now learned the state called those same results "Negative" during the peak of the pandemic.
In a statement, the FDA explained: "According to the authorized labeling for the PerkinElmer test, patients' samples that have Ct values (less than) < 42 … should be reported as positive."
Simply put, the Ct value is a measure of viral load in the sample. More specifically, it is the number of cycles necessary for the PCR machine to identify the virus. The lower the number, the more likely the result is positive.
The FDA authorized instructions for the PerkinElmer test requires that any Ct values below 42 must be reported as "Positive."
Instead, the California Department of Public Health told CBS13 that it reports results between 37-42 as "Inconclusive."
We've since learned the state lab called those same results "Negative" until mid-December and then started reporting them as "Presumptive Positive" to patients, but "Inconclusive" to the state's official database.
SAME PATIENT, SAME LAB, DIFFERENT RESULTS
For example, CBS13 Investigative Reporter Julie Watts obtained a copy of the results for her son's COVID tests as they were officially reported to the CalREDIE database - the California Reportable Disease Information Exchange.
He received three different results from the state lab for roughly the same measure of virus (Ct 39) on two different tests: "Negative," "Presumptive Positive" and "Inconclusive."
Meanwhile, public health labs across the state tell CBS13 they would have reported his results on both tests as "Positive" because they are using the test according to the FDA authorization.
Same patient, same levels, same test, four different results.
In November, when his lab report indicated that he had a Ct value of 39.8, the state lab reported him as "Negative" both to the CalREDIE database and on the patient result letter sent to Watts.
However in January, when he had a Ct value of 39.04, the state lab reported him as "Inconclusive" to the CalREDIE database, but told Watts that her son was "Presumptive Positive".
"That's not normal," said UCSF Lab Director Dr. Tim Hamill, who previously chaired the state's lab advisory committee (CLTAC).
Hamill was one of several lab directors and industry experts who took issue with the practice of reporting one thing to the patient and something different to the official database.
"That's not what a lab should be doing," Hamill said. "They should be reporting it in one fashion to all individuals."
ALSO READ: 'Inconclusive' COVID Results Should Be 'Reported as Positive'
Other lab directors we spoke with questioned whether it could be a tactic to keep the official COVID positivity rate down. None of the other labs we contacted use the term "Presumptive Positive."
One lab director who spoke on background called it a "win-win for the state," noting that telling patients they're "Presumptive Positive" while officially reporting them as "Inconclusive" allows the state to keep the positivity rate down while encouraging people to act as if they are positive.
Several lab directors declined to speak on the record citing the potential repercussions of publicly criticizing the state lab.
In a statement California Department of Public Health (CDPH) said:
"Under non-pandemic conditions, laboratories have more time to collect data to confirm test performance characteristics. In the case of the CDPH Valencia Branch Laboratory, implementing a new test in a new facility in response to urgent unmet needs, the decision was made to be more conservative when the lab was first opening. In order to provide clearer direction to the patient, patient result categories were updated as more information became available to include a presumptive positive category and recommend to those patients a second specimen collection to confirm if the individual is either positive or negative, which is the best practice for borderline test results."
Whistleblowers inside the state lab argued that instead of "experimenting with patient samples" during the peak of the pandemic, the lab should have been following the FDA-authorized instructions for its own PerkinElmer test.
CDPH explained that PerkinElmer's test is "is highly sensitive and able to detect lower levels of viral load than other tests on the market." As a result, they said they report those "lower level" results as "Inconclusive."
However, several other labs that are using the same "highly sensitive" test are reporting those same "lower levels" as "Positive" according to the FDA-authorized instructions.
SAME TEST, DIFFERENT LABS, DIFFERENT RESULTS
Several county public health labs, and private labs, across the state are also using the PerkinElmer test.
Of the eight labs that responded to our requests for information about how they report the "lower levels" (Ct 37-42):
- 3 labs say they now call these results "Inconclusive" - including the CDPH PerkinElmer lab.
- 3 labs still call them "Positive" - as required by the FDA authorization.
- 2 labs say they also use a different company's test and release those results instead of the PerkinElmer's.
One county public health lab tells us they use the PerkinElmer test in-house, according to FDA instructions, and report "lower-levels" as "Positive." However, they also send some of their tests to be processed at the state lab where those same "lower-levels" would be reported as "Inconclusive".
Same test, the same measure of the virus, same patient population, different results.
"You'd like to have everybody on the same page with how to interpret that test," Dr. Hamill said. But, he explains, that is not always the case.
MORE ACCURATE RESULTS?
In this case, while PerkinElmer has a $1.7 billion contract to run the state's COVID testing lab, PerkinElmer is also a manufacturer that sells its test to other labs nationwide.
Before PerkinElmer could sell the test, they had to get an Emergency Use Authorization (EUA) from the FDA. The EUA includes detailed instructions on the positivity cutoff, which was based on PerkinElmer's submitted testing data.
Labs that purchase PerkinElmer's FDA-authorized test must call all values below Ct 42 "Positive" based on that submitted data, unless they do their own in-depth study, called a "validation," that proves another result is more accurate.
Then, the test becomes a Lab Developed Test (LDT) which is not authorized, or required to be approved, by the FDA and can only be used in that one lab.
READ MORE: 'Inconclusive' COVID Results Should Be 'Reported as Positive'
The PerkinElmer public health lab claims it did a validation that indicates "Inconclusive" is more accurate than "Positive" for Ct values between 37-42 because "the test is able to detect some virus, but not enough to clearly label the specimen as positive."
However, "Inconclusive" is not an option for the other labs using PerkinElmer's FDA-authorized test.
The test has not been updated to include "Inconclusive" results even though the EUA requires PerkinElmer to notify the FDA of "deviations from the expected performance of the test."
CONTINUING COVERAGE: CBS13 Investigates Problems at California's COVID Testing Lab
One other lab that calls the "lower level" results "Inconclusive" says they have done their own validation.
Another lab admits they haven't done a validation but says they call "lower level" results "Inconclusive" based on past patients with those levels who "showed no COVID-19 symptoms or contact history."
Still other labs tell CBS13 that they are aware of the concerns about the "lower level" results but, since they have not done their own in-house validation, they are required to follow the EUA and release the results as "Positive."
CBS13 asked, "[i]f CDPH, PerkinElmer and several public health labs believe that "inconclusive" is a more accurate result than "positive," why hasn't PerkinElmer updated the EUA so that all labs are reporting the same results?"
Neither CDPH nor PerkinElmer provided a direct response.
The FDA tells CBS13:
"The FDA is aware of the issues raised regarding the Valencia lab and PerkinElmer test result concerns and are looking into this issue. We will keep the public informed as appropriate if any further actions are taken."
FALSE POSITIVES?
So, is it possible that labs are reporting false positives when using PerkinElmer's FDA-authorized test?
"My hypothesis is that they're finding the actual genomic information of the virus," said Nevada state lab director Dr. Mark Pandori. "When you're dealing with specimens in this range, they're so weakly positive it doesn't represent a public health or medical threat, necessarily."
Pandori explained that PCR tests in general are so sensitive that they may detect insignificant amounts of virus.
PerkinElmer boasts its test is the most sensitive.
"I would say [that extreme sensitivity] is the exact source of this confusion," said Pandori.
According to the CDC, Ct values can be "interpreted as positive or negative but cannot be used to determine how much virus is present."
Similarly, the FDA says it's not clear "whether or not particular Ct values correlate with a person being ... infectious."
In the meantime, people who are getting "Positive" results based on weak Ct values have real-world consequences.
"Should we be resulting those as positive?" Watts asked.
"As we come out of the pandemic, it's that question that we really have to grapple with," Pandori said.
Dr. Hamill cites research that suggests it's better to err on the side of false positives early in the pandemic "so that few, if any cases are missed."
However now, as case counts diminish and vaccine rates rise, "a less sensitive test with high specificity may be more desirable," he said, to avoid penalizing people with trace amounts of the virus who are not infectious.
Incidentally, the state lab appears to have done the opposite by reporting "lower levels" as negative during the peak of the pandemic, then later calling those same results "presumptive positive/inconclusive". Though notably, presumptive positive patients do not face the same quarantine requirements as those who receive a "Positive" diagnosis.
YOUR RIGHTS TO YOUR LAB RESULTS
As negative tests are increasingly required for travel and return to work or school, any "Positive" test can have significant consequences -- even if, like Tim, you test "Negative" the next day.
"That affected so many people that I have everyday contact with," Tim said. "People couldn't work."
Tim doesn't know what his Ct value was. He's been trying to get a copy of his detailed lab report for more than a month.
"I'm told that I'm not entitled to see it," he said. "If they're going to tell somebody you're positive for this disease, then you should be able to provide us with the information."
Despite reaching out to the county and the lab, Tim has only received the initial email notifying him of his "Positive" result.
California Health & Safety Code section 123110 permits inspection of patient records within five working days after receipt of a request, and requires health care providers to provide copies of patient records within 15 days after receiving a request.
However, as we learned, it can be next to impossible to get a copy of your lab report from the CDPH-PerkinElmer lab.
CDPH says patients can access test records "through the organization that provided their results" but that didn't work for Tim.
CDPH says people can also submit personal information records requests using the forms located here: Your Privacy Rights (ca.gov).
It's been over a month since Watts put in a request to obtain her son's lab report through the CDPH privacy office and the state has yet to provide those reports.
Watts was able to obtain some of the records through her county health department, and other records from a third-party contractor, but the state has not provided the official lab reports that are supposed to include the reporting ranges, disclaimers and other information about the test used.
It's important to note that the Ct values are not necessarily included on the lab reports. However, it appears the state lab did report them for some tests.
CDPH told CBS13 that, "it would be a regulatory violation" to report Ct values, pointing to this Q&A from an industry group.
However, various lab directors and industry experts tell CBS13 there is no regulation that prevents a patient from obtaining their Ct value.
Considering the questionable ranges for the PerkinElmer test, and the fact that the state lab changed the positivity cut off several times, Tim believes he should be entitled to see his full lab report, including his Ct values.
"That's 10 days of my life I'm never going to get back," he said. "I just wish that the state of California would be more forthcoming."
NOTE: This story originally aired on 3/22/21 but was published online with additional information on 3/24/21.
WATCH THE CBS NEWS SACRAMENTO SPECIAL REPORT
THE COVID LAB: State Secrets Exposed
This 30-Minute Special Report is the culmination of 14 months of reporting that prompted state and federal investigations, resulted in two new pieces of legislation, and shined a spotlight on shocking public health failures that it appeared regulators tried to hide.
Following these reports, lawmakers introduced legislation that is intended to protect whistleblowers and ensure accountability and transparency long after the pandemic is over. The state ultimately terminated its $1.7B COVID contract with PerkinElmer.