State Inspections Confirm Whistleblower Allegations At Troubled California COVID Lab
SACRAMENTO (CBS13) - The California Department of Public Health (CDPH) released its long-overdue report on state inspections of California's troubled COVID testing lab, following a CBS13 whistleblower investigation. Inspectors confirmed the whistleblowers' allegations and found the lab "pose(d) immediate jeopardy to patient health and safety."
Along with inspection records released Monday, CDPH issued a summary that downplayed the findings and misconstrued some whistleblower allegations. However, the actual inspection records tell a very different story.
Lab inspectors issued scathing reports following the state's routine initial inspection and the whistleblower complaint investigation. Both inspections found the lab "caused, is causing, or is likely to cause, at any time, serious injury or harm, or death" to Californians.
Instead of pausing testing or warning the public, the state allowed the lab to continue processing patient samples and concealed the risks as problems continued for at least six months and the lab began contracting with schools across the state.
Inspection records confirm whistleblower allegations that:
- Lab practices "pose(d) immediate jeopardy to patient health and safety"
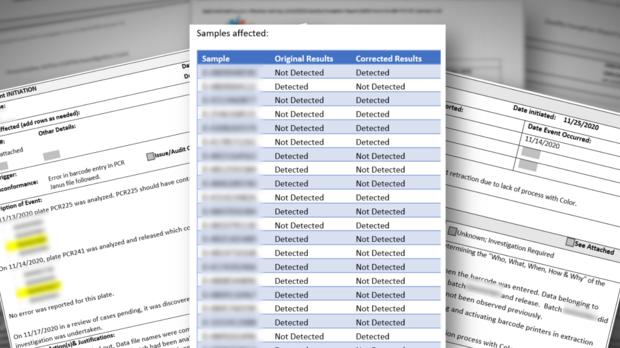
- When the lab discovered it reported wrong results, it did not immediately notify patients
- There were widespread contamination concerns
- Roughly half the lab's employees did not have documented competency
- Lab errors were effectively concealed by miscategorizing errors as problems with the sample
- The lab voided PerkinElmer's FDA Emergency Authorization by repeatedly making unauthorized changes to the test
- The lab used its version of the test on patients for months before properly validating that the results were accurate
Records also reveal:
- Inspectors found that lab director, Dr. Adam Rosendorff, was not qualified to run the lab "based on the severity of deficiencies" found. Rosendorff is the former lab director of Elizabeth Holmes' disgraced finger-stick blood-testing company, Theranos.
- The lab did not properly report delayed results. Their contract mandates a 48hr testing turnaround time.
- Deficiencies had not been corrected months after CDPH and the Newsom Administration claimed that they were "resolved."
- The lab continued to face sanctions in October, ten days before its $1.7B state contract was renewed.
*Note this is a partial list of deficiencies, failures and patient dangers that may be updated as we continue to review inspection records.
During the Feb 7th surprise inspection, prompted by the whistleblower complaints, inspectors confirmed the allegations and found problems so egregious that they were forced to notify federal regulators, stating in a notice:
"Because of the seriousness of these deficiencies, your laboratory no longer meets the requirements to perform testing under the Health and Safety Code. Based on the finding of immediate jeopardy, this office has contacted the Centers for Medicare & Medicaid Services (CMS), and has notified them of our determination of non-compliance.
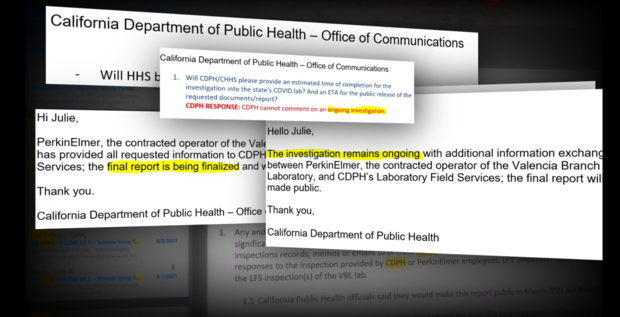
Two weeks later, CDPH and PerkinElmer claimed the "serious deficiencies" had "long since been resolved." We now know that simply wasn't true.
Not surprisingly, the agency did not issue sanctions against its own lab Monday. Instead, in its summary of the investigation, CDPH concluded, "(t)his blueprint can serve as a model for other states, and the federal government, in how to scale testing."
If it weren't for brave whistleblowers risking their careers in the name of public health, the public may never have learned of these shocking public health failures at California's COVID testing lab.
UPDATE - Please See Updated Report here: