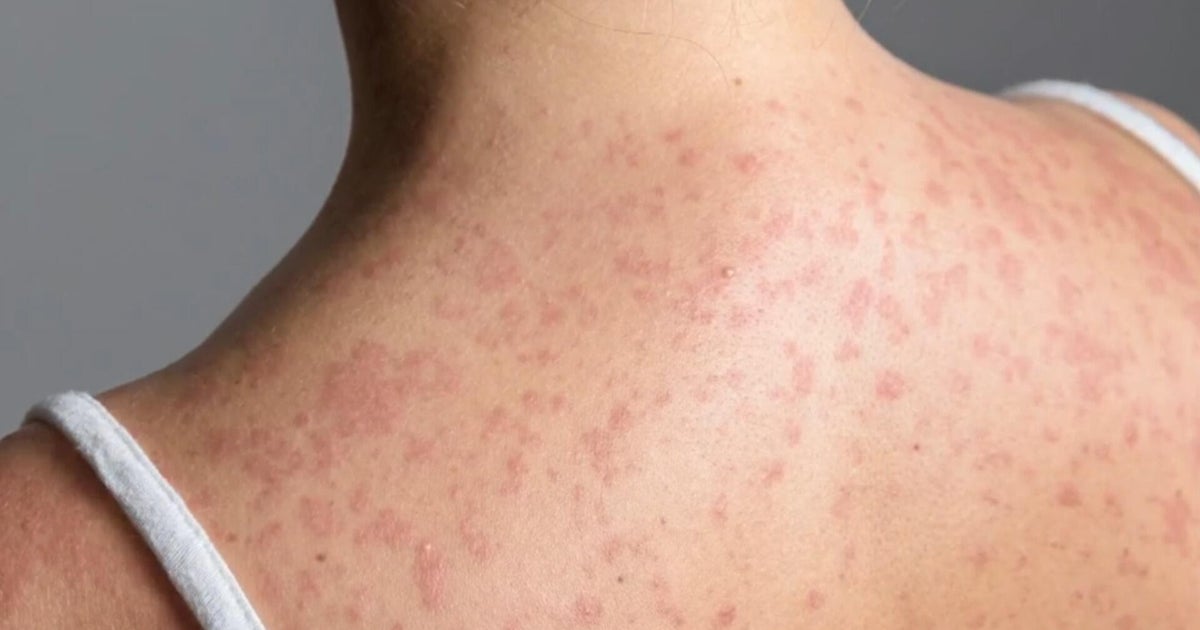
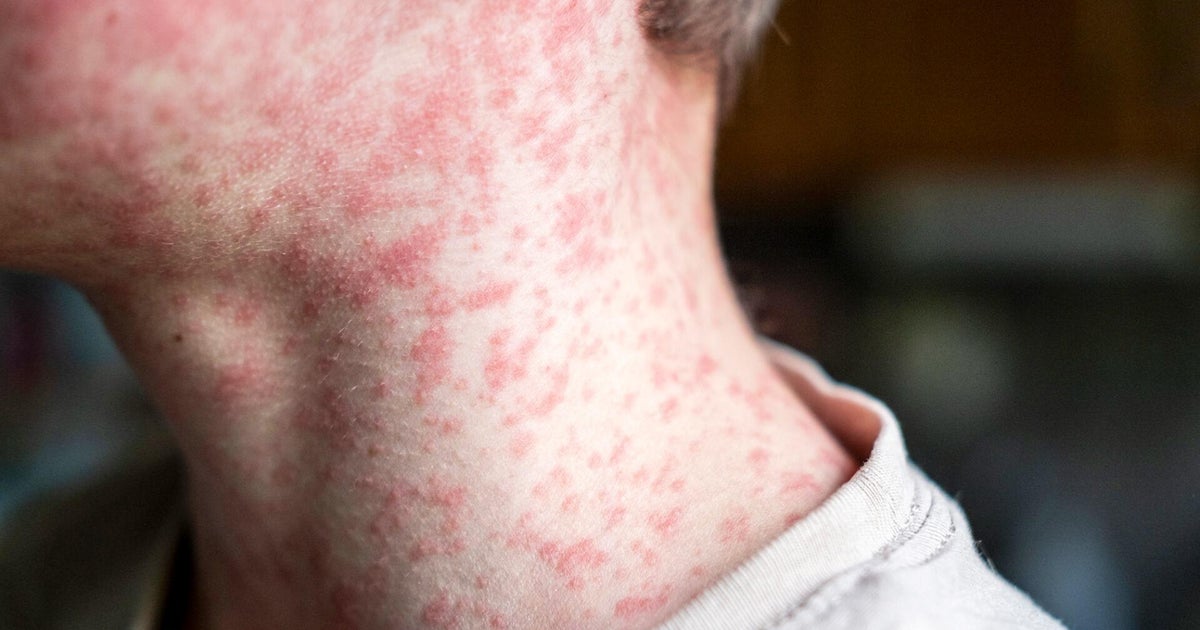
U.S. confirms first two cases of monkeypox in children — one case is in California
Health officials have confirmed the first two U.S. cases of monkeypox in children, the Centers for Disease Control and Prevention announced Friday.
Both cases are "likely the result of household transmission" and "had no contact with each other," the agency said in a statement.
One is a toddler who lives in California and the other is in an infant who is not a resident of the U.S. and was "transiting through" the Washington, D.C. area when the test was done.
"We became aware of these cases just this week, and we've been working with the jurisdictions to understand more about these cases," the CDC's Jennifer McQuiston told reporters on Friday.
CDC Director Dr. Rochelle Walensky first disclosed news of the cases at a virtual event with The Washington Post on Friday, saying that both children "are doing well."
Children, especially those under 8 years old, are among those the Centers for Disease Control and Prevention warns are at "especially increased risk" for severe monkeypox disease.
Last week, CDC officials told reporters that at that point, they were only aware of monkeypox cases in adults. But the agency acknowledged that state and local health authorities had only relayed additional demographic information to them for less than half of all tallied cases.
The agency is also now aware of at least eight cases in people who identify as cisgender women, McQuiston said. Most cases so far have been among men who have sex with men.
"There is no evidence to date that we're seeing this virus spread outside of those populations to any degree, and I think that the primary drivers for this infection in the U.S. remain in the gay, bisexual, and men who have sex with men communities right now," McQuiston added.
As of Friday, the CDC had tallied a total of 2,891 cases of monkeypox in the U.S. across 44 states, as well as the District of Columbia and Puerto Rico.
While the virus has led to many adult patients enduring pain and sometimes severe complications, many of the cases have so far resolved after several weeks without intensive treatment or hospitalization.
But health authorities warn that monkeypox may pose greater dangers to young children.
In countries that have seen an endemic spread of monkeypox before 2022, the World Health Organization warns that young children have died at higher rates from the disease.
During the current outbreak, a handful of countries have also spotted cases of monkeypox in children under 18 years old.
The CDC's European counterpart tallied at least five cases on Wednesday. Authorities in Spain's capital announced on Wednesday that they had detected a case in a 7-month-old baby who likely caught the virus from their parents.
In the Netherlands, doctors reported they were unable to identify how a boy under 10 years old had caught the virus. No secondary cases were identified from the infection.
As is the case with adults, medicines like the antiviral tecovirimat or TPOXX are available for treating monkeypox cases in children and have been safely given to children in the past. However, the CDC says no clinical studies have specifically investigated use of the antiviral in children.
The agency said that both children are being treated with tecovirimat.
For vaccination, Food and Drug Administration has only formally approved the Jynneos monkeypox shots for use in adults. However, federal health officials have said the Biden administration has worked out arrangements to be able to offer doses for children in the current outbreak.
In June, CDC officials said that they had offered doses of the Jynneos vaccine to at least one pediatric patient. This week, a hospital in New Jersey announced they had facilitated vaccination of a 3-year-old who had been exposed to a positive case.
CDC scientists have estimated that monkeypox symptoms during the current outbreak take a little over a week on average to develop, following exposure to an infected person.
During that incubation period, the CDC says taking the two-dose monkeypox vaccine can still reduce the severity of the disease or even prevent it from developing.
"CDC recommends that the vaccine be given within 4 days from the date of exposure in order to prevent onset of the disease. If given between 4–14 days after the date of exposure, vaccination may reduce the symptoms of disease, but may not prevent the disease," the agency says in its guidance.
A spokesperson for the Food and Drug Administration declined to confirm how many requests the agency has granted for use of the vaccine in children.
Bavarian Nordic plans to collect data based on the CDC's use of the vaccine, a company spokesperson confirmed.