Cheaper pills? 7 top-selling drugs set to go generic
Relief may be in sight for people having trouble paying for costly prescription drugs. Several top-selling drugs are set to lose patent protection over the next year. "My estimation is at least 15 percent of the population is currently using one of the drugs whose patents will expire in 2011 or 2012," Joel Owerbach, chief pharmacy officer for New York's Excellus Blue Cross Blue Shield, told the Associated Press.
Generic drugs cost 20 to 80 percent less than brand name equivalents, and can cost as little as $5 on some insurance plans.
Keep clicking to see 7 top-selling drugs set to expire, as listed by pharmacy benefits company Medco...
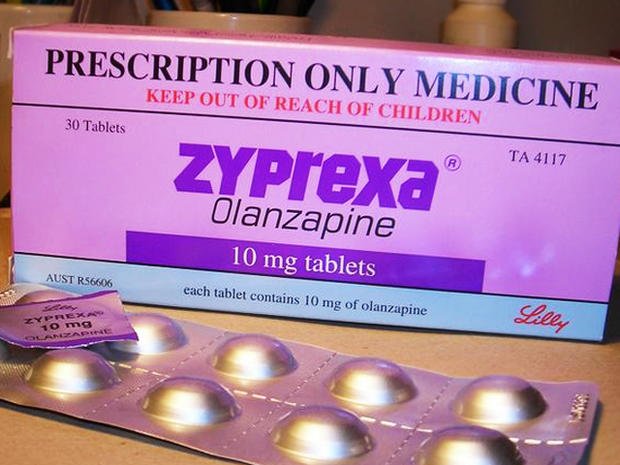
Zyprexa
Zyprexa is made by Eli Lilly to treat schizophrenia and bipolar disorder in adults and teens over 13 years old. Its patent is set to expire this October.
Lexapro
Lexapro, manufactured by Forest Laboratories, is taken once a day to treat depression and anxiety. Lexapro's patent will expire in March 2012.
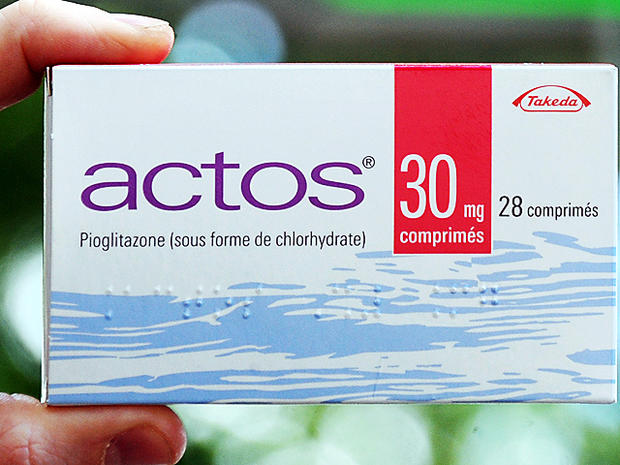
Actos
Actos is Takeda's popular type 2 diabetes drug that controls blood sugar levels. The patent is set to expire in August 2012.
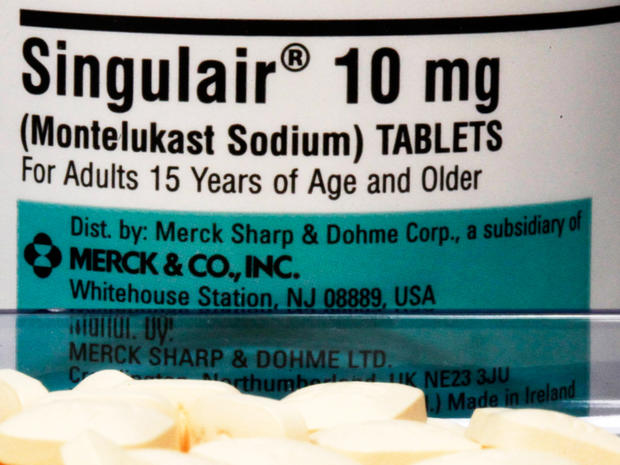
Singulair
Singulair is made by Merck to relieve asthma and allergy symptoms like difficulty breathing, chest tightness, sneezing, wheezing and coughing. Singulair's patent will run out in August 2012.
Seroquel
Seroquel is an AstraZeneca drug that treats depression, bipolar disorder, and schizophrenia. It's set to go generic in March 2011.
Plavix
Plavix, a blood thinner that's marketed by Bristol-Myers Squibb and Sanofi-Aventis, is taken by 1.4 million Americans to prevent heart disease and stroke. It costs about $200 for a month's supply but will be much cheaper when its patent expires in May 2012.
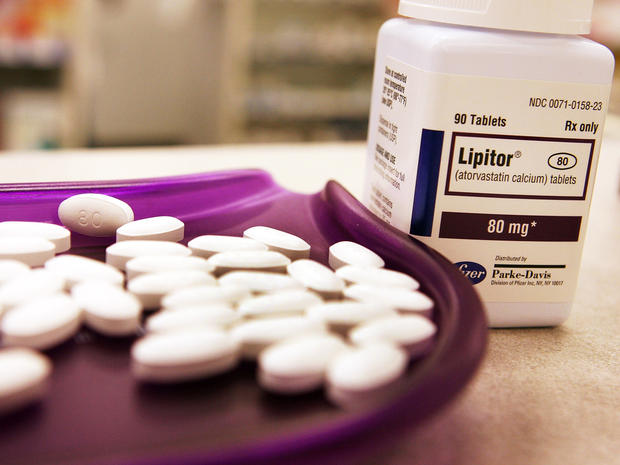
Lipitor
Pfizer's Lipitor is one of the most commonly prescribed drugs in the U.S, taken by almost 4.3 million Americans. It's a once daily statin that's taken to reduce the risk of heart attack and stroke. Lipitor currently costs about $150 a month, but could plummet as low as $5 on some drug plans when its patent expires in November 2011.