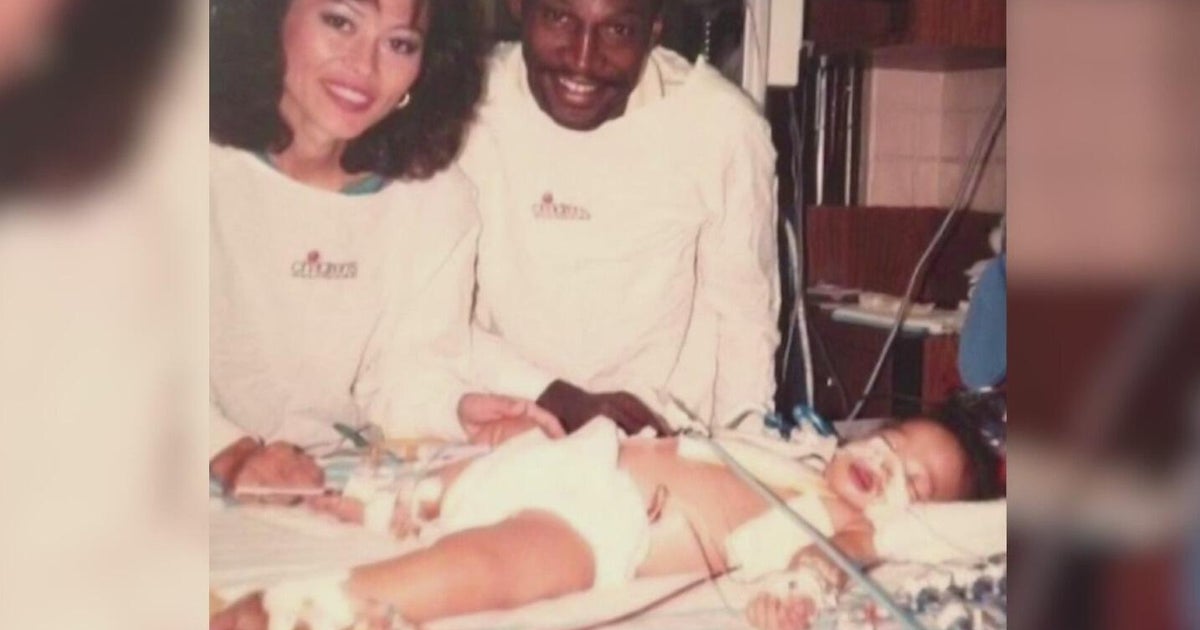
Nicole Audia says Johnson & Johnson vaccine sent her health into a tailspin
NEW YORK - The FDA announced strict new restrictions on the Johnson & Johnson vaccine due to the risk of rare and life-threatening blood clots.
Now it's only recommended for people who can't get another vaccine or otherwise won't be vaccinated at all.
CBS2's Jessica Moore spoke with a woman who says she wishes she knew about the side effects sooner.
From smiling with her family to an agonizing hospital stay, 51-year-old Nicole Audia says the Johnson & Johnson vaccine sent her into a physical tailspin.
"I was in the hospital for six weeks, out of work for 10. My heart will never be the same, and that stinks," Audia said.
Audia, a mother from Hopewell Junction, says two weeks after getting the J&J vaccine she had a stroke. Her hair fell out in clumps, and doctors injected her with massive amounts of steroids to help calm her body's extreme reaction.
"When you're in the hospital, was there any point where you thought, 'I may not make it out of here?'" Moore asked.
"Yeah. I had a fever for five weeks straight," Audia said.
According to the CDC, more than 18.7 million doses of the J&J vaccine have been administered in the United States. Sixty cases of a rare blood clot condition called TTS have been confirmed, including nine deaths.
The FDA is now recommending people not get the single shot vaccine unless it's the only one available or they won't otherwise get vaccinated.
"What you're hearing from the FDA is we do know this a very, very rare side effect called TTS. And what this is, is a clot that some people develop it, you know. It happens about three in a million," said White House COVID-19 response coordinator Dr. Ashish Jha.
"When you hear now that the FDA is recommending most people not get the J&J vaccine, how does that make you feel?" Moore asked.
"Like a day late and a dollar short. I feel like I really wish I knew that," Audia said.
The FDA is now strongly suggesting people get Pfizer or Moderna, begging the question: Was enough testing done on the J&J shot before it received FDA approval?
"A bad side effect from this vaccine is far rarer than the bad side effect from taking a daily aspirin. So it's not like we're talking about something that would have been easily picked up in a clinical trial," Jha said.
Jha says people who got the J&J vaccine a while ago shouldn't worry. The risk of blood clots and complications vanishes within the first few weeks after injection.
"I want my hair back. Aside from that -- it's coming back -- I just want my life to be where it was. It never will be," Audia said.
Audia says she still strongly supports getting vaccinated, but wishes she would've known the risks before taking this particular shot. She says the CDC and J&J are aware of her case and are investigating.
"There is no greater priority than the safety and well-being of the people we serve. With billions of people receiving COVID-19 vaccines around the world, rare adverse events can occur, and it's why no-fault compensation systems, such as the U.S. Countermeasures Injury Compensation Program are so important," a spokesperson at Janssen sadi.