U.S. meningitis outbreak claims four more lives
Four more deaths have been linked to a meningitis outbreak caused by contaminated steroid injections made by a specialty pharmacy, U.S. health officials announced Wednesday. That brings the nationwide total to 19 deaths.
A Centers for Disease Control and Prevention (CDC) update listed 247 reported infections in 15 states on its web site. The agency had previously reported 233 infected Americans including 15 deaths in 15 states.
Two of the reported cases may be joint infections from steroid shots given in peripheral joints such as the knee, hip, shoulder and elbow. No deaths have been associated with peripheral joint infections, the CDC said.
One new death was reported in both Florida and Virginia, and two more deaths were reported in Tennessee, the state with the most reported infections with 61 cases.
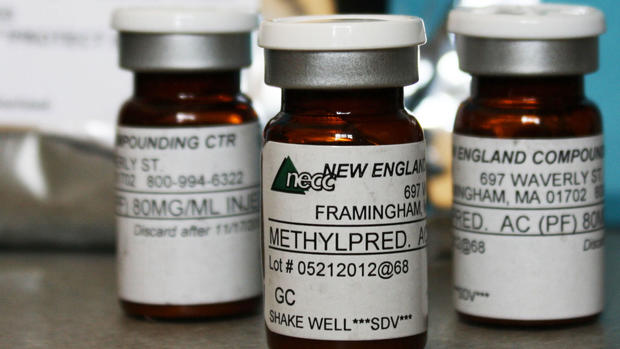
The outbreak has been tied to methylprednisolone acetate steroid shots used for back pain made by a specialty pharmacy, New England Compounding Center, in Framingham, Mass. The center is currently under investigation by federal agencies.
FDA officials have said three potentially contaminated lots of the injections had over 17,000 vials in them. Up to 14,000 patients may have received the injections.
Officials have also reported infections in two people who got different drugs made by the company. One case is possible meningitis in a patient who got a spine injection of another type of steroid, triamcinolone acetonide. The agency also learned of one heart transplant patient who got fungal infections after being given a third company product, cardioplegia, during surgery.
- Lawmakers call for probe of meningitis-linked firm
- After 15 dead, feds search Mass. firm in meningitis case
- What contaminated the shots that spread meningitis?
Criminal investigators from the Food and Drug Administration have visited the New England Compounding Center.
FDA spokesman Steven Immergut said Tuesday the agents' work at the company is part of the investigation into the outbreak.
The Department of Justice confirmed Tuesday that it had opened an investigations into the NECC, but Massachusetts U.S. attorney Carmen Ortiz added it was "entirely premature to suggest what the results" would be.
Company attorney Paul Cirel says it's "difficult to understand the purpose" of the FDA search. He says the company has been clear it would voluntarily provide anything investigators requested. He says the company will continue to cooperate.