Pharmacy head pleads Fifth at meningitis outbreak hearing
Updated 5:10 p.m. ET
WASHINGTON Barry Cadden, the owner and director of the specialty pharmacy tied to a deadly fungal meningitis outbreak, declined to testify Wednesday morning before a congressional committee investigating the matter.
Cadden, co-founder of the New England Compound Center, told lawmakers he would use his Fifth Amendment right to not answer questions in order to avoid self-incrimination.
After repeated questioning by House lawmakers, Cadden told the House Energy and Commerce Committee: "Under advice of counsel, I respectfully decline to answer under basis of my constitutional rights and privileges, including the Fifth Amendment."
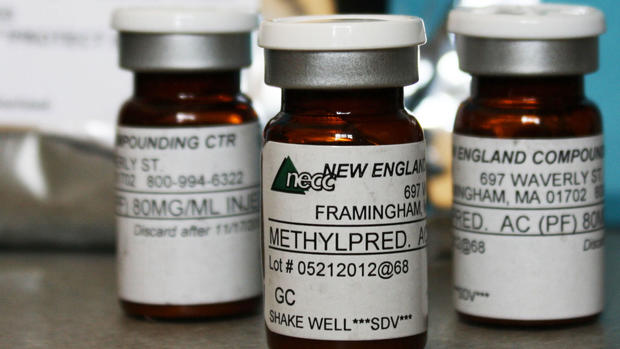
Lawmakers continued to ask Cadden questions about the contamination of steroids used to treat patients -- many for back pain -- that has sickened nearly 430 people with fungal meningitis and caused 32 deaths. Another 10 patients have peripheral joint infections from injections.
Arecent investigation of the NECCfacility in Framingham, Mass., found numerous sterility issues, including bacterial contamination and mold growth in areas where drugs are prepared. Compounded drugs are supposed to be prepared in temperature-controlled clean rooms to maintain sterility.
The NECC has been closed since early last month, and Massachusetts officials have taken steps to permanently revoke its license. The pharmacy has recalled all the products it makes, including about 17,700 single-dose vials of a steroid that up to 14,000 patients my have received.
Fungal meningitis causes inflammation of the lining of the brain and spinal cord.
Republicans lawmakers, who make up the majority of the House, focused on NECC's history of troubles, when questioning why regulators at the Food and Drug Administration and the Massachusetts board of pharmacy did not take action against the pharmacy years earlier.
"After a tragedy like this, the first question we all ask is: could this have been prevented?" asked Rep. Cliff Stearns, R-Fla. "After an examination of the documents provided by the Massachusetts Board of Pharmacy and the FDA - the answer here appears to be yes.
A timeline assembled by the Republican staff shows that the FDA inspected NECC three times since 2002 for sterility issues. The Massachusetts board of pharmacy investigated at least 12 separate complaints involving the pharmacy, dating back to its founding.
"I was stunned and angered to learn that an inspection of the NECC by the FDA and the Mass Board over 10 years ago identified contamination in the very same drug at issue in the current outbreak," said Rep. Fred Upton, R-Mich., who chairs the Energy and Commerce committee.
FDA Commissioner Margaret Hamburg told lawmakers that the problems uncovered in inspections were "very serious," but that the agency was obligated to defer to Massachusetts authorities, who have more direct oversight over pharmacies.
"The challenge we have today is that there is a patchwork of legal authorities that oversee the action we can take," said Hamburg, who was nominated to head the FDA by President Obama in 2009.
Hamburg asked lawmakers Wednesday in prepared testimony to give her agency more authority and funding to oversee compounding pharmacies.
"In light of growing evidence of threats to the public health, the administration urges Congress to strengthen standards for non-traditional compounding," Hamburg told lawmakers.
Compounding pharmacies traditionally fill special orders placed by doctors for individual patients, turning out a small number of customized formulas each week. They are typically overseen by state pharmacy boards, though the Food and Drug Administration occasionally steps in when major problems arise. Some pharmacies have grown into much larger businesses in the last 20 years, supplying bulk orders of medicines to hospitals that need a steady supply of drugs on hand.
She said Congress should put in place a two-tier system in which traditional compounding pharmacies continue to be regulated at the state level, but larger pharmacies would be subject to FDA oversight.
Cadden's appearance before the committee came immediately after the widow of a longtime Kentucky judge who was the first confirmed victim of the outbreak.
Speaking without notes, Joyce Lovelace told lawmakers her story of more than 50 years of marriage to 78-year-old Eddie C. Lovelace, who was a circuit judge until he died Sept. 17 at Vanderbilt University Medical Center.
She asked lawmakers to implement laws to police companies like the New England Compounding Center, which distributed steroids that tested positive for contamination.
"My family is bitter, we are angry, we are heartbroken and devastated. I come here begging you to do something about the matter."
- FDA finds contamination, insects and flying bird at Ameridose
- Ameridose, tied to pharmacy in meningitis outbreak, lays off staff
- President of firm linked to meningitis outbreak to testify before Congress
CBS Evening News correspondent Jim Axelrodreported on Tuesday that Rep. Stearns had expected Cadden to take the Fifth and stay silent.
One incident he was hoping to get answers on occurred in September 2004, when FDA investigators asked Cadden if he had "Trypan Blue" solution on hand. The product is not approved by the FDA.
The CBS News investigative team's Laura Strickler reports that investigators then found a drawer labeled Tryban Blue that contained 189 vials of the drug. When presented with their findings, Cadden reportedly became "indignant" and told the company's co-owner Greg Conigliaro to no longer answer the investigators' questions.
Another company owned by Cadden and Conigliaro, Ameridose, wasalso discovered to have sterility issues during a recent inspection when investigators found insects and birds inside the facility. The company will be shut down until Nov. 19 at the earliest, and has had to lay off 650 employees and an additional 140 employees at its marketing and support arm because of the extended closure.