She signed up for a complicated clinical trial. It may cure her lupus.
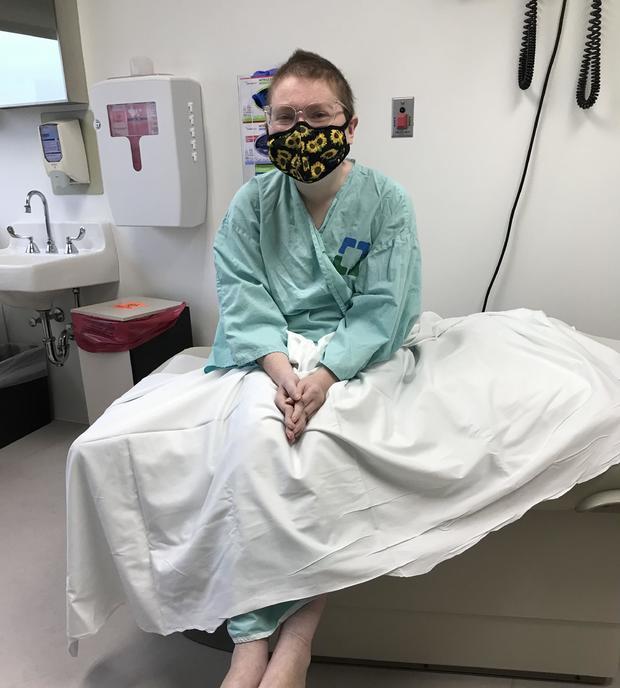
West Virginia teacher Sierra Butler spent years of her life dealing with symptoms she couldn't connect including fatigue, joint pain and weight loss. In 2020, she lost over 100 pounds — and the ability to walk.
Finally, she got a diagnosis: lupus, a chronic autoimmune disease that causes pain and inflammation in the body.
In some people, lupus can affect the heart and cardiovascular system. That's what happened to Butler: During the summer of 2020, she was in and out of the hospital, sometimes staying in the intensive care unit for days at a time. One night, she had an overwhelming sense of impending doom and called 911.
While hospitalized, she was tested for C-reactive protein, a blood marker that indicates inflammation. A healthy person will have a CRP level of 0.2 or 0.3. Butler's level was over 100, and she was immediately admitted to the cardiac ICU that she had left just days before.
Butler was then airlifted to another hospital in the state. While in the air, she went into cardiac tamponade, a condition where the heart cannot beat properly. Emergency surgery saved her life but caused her and her medical team to reevaluate how they were treating her lupus, which was resistant to typical treatment options.
"My cardiologist called me and he apologized if it was overstepping, but he asked for my permission to refer me to the Cleveland Clinic," the 27-year-old said. "He said that he hated to see a young person with a healthy heart going through cardiac issues because my lupus was not being controlled. ... He was fearful that if I did not get my lupus controlled quickly, then I would develop very serious, irreversible cardiac problems."
Butler began seeing doctors at Cleveland Clinic in 2021. She went from being bald and in a wheelchair with limited mobility to being able to walk and drive as doctors perfected a drug regimen that worked for her. She and her husband even welcomed their first child in November. In January, she received a new opportunity: She could be one of just two people in a complex clinical trial that would use CAR-T therapy to treat treatment-resistant lupus.
What is CAR-T therapy, and how can it treat lupus?
CAR-T therapy, or chimeric antigen receptor T-cell therapy, essentially removes a patient's white blood cells and genetically modifies them to recognize and attack problematic cells. It's most commonly used to treat cancer, according to Dr. Emily Littlejohn, Butler's rheumatologist at the Cleveland Clinic.
The first study researching CAR-T therapy as a lupus treatment was published in 2021. Five people who had not had benefits from other lupus treatments received the therapy, which essentially uses a patient's "own cells to find lupus in the body and kill the cells that are driving the lupus activity," according to Littlejohn. The patients did "very well" and remain in remission, Littlejohn said.
"Their lupus completely ceased to exist. Their arthritis, the fatigue, all of these other manifestations essentially disappeared, and they were able to come off all of their medications," Littlejohn said. "And that's pretty amazing in that lupus is a disease where we don't ever take people off their medications. That's really unheard of in this space."
To treat lupus, the T-cells are genetically modified to attack the overactive B-cells that create inflammation in a patient's body, said Dr. Anca Askanase, the director of the Columbia University Lupus Center, who was not involved with the Cleveland Clinic trial or Butler's treatment. Askanase said that with CAR-T therapy, the troublesome B-cells are "completely destroyed" by the genetically modified cells, allowing the patient to enter remission.
A complex clinical trial
Butler and her husband had read about CAR-T treatments for lupus being tested overseas since 2020, but had never been optimistic that she would be able to try them. When Littlejohn asked if she would be interested in participating in the Cleveland Clinic trial, she could barely contain her emotions.
"My husband and I were in the parking lot just sobbing, because we were told it could be decades before this would even potentially
be in the United States," said Butler. "From there, we just kind of hit the ground running and I haven't looked back. My entire life since that phone call has centered around the trial."
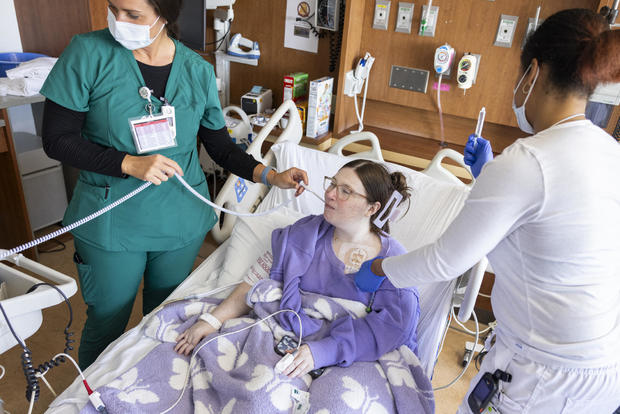
That began a monthslong, complicated process, starting with what Butler called "the most blood work I've ever had in my life" as part of making sure she met the trial's requirements. In April, she went off her medications and underwent leukapheresis, where white blood cells were removed. The white blood cells were then sent to a lab and trained to attack Butler's B-cells. A week later, the clinic confirmed that the step had been successful. With that confirmed, Butler and her family relocated to be near the Cleveland Clinic for the next steps of the trial.
In May, Butler began chemotherapy to "wipe out the immune system" to remove as many existing B-cells and white blood cells as possible before the modified cells were put back into her body.
Butler remained in the hospital for two weeks so that her immune system could recover and so she could be watched for possible side effects. Once she was discharged, she returned to the clinic multiple times a week for bloodwork and other tests to confirm the process had worked.
On June 3, 28 days after the cells had been transplanted, Butler had another round of bloodwork to confirm that the therapy was continuing to control her lupus. An exam was also conducted to see if Butler had physical symptoms like joint swelling or rashes. Littlejohn said that just a month out from treatment, Butler was "doing well."
"Her blood work looks great. Her lupus looks much more well-controlled," Littlejohn said. "We expect it's going to keep getting better. It's a little soon to see the full results of the CAR-T treatment, but we expect things to keep improving. She feels better. She has less joint pain, less rashes, less oral ulcers and canker sores, but also serologically, in her blood, there'll be less lupus activity."
The future of CAR-T therapy for lupus treatment
Since the German study was published, there has been an explosion of interest in treating lupus with CAR-T therapy, Askanese said. Thirty-nine patients worldwide have enrolled in clinical trials, she said. The first trial in the United States for children with lupus was recently approved by the Food and Drug Administration.
While the results of trials around the world have been promising, Littlejohn and Askanase both said there's a long way to go before CAR-T therapy is more widely used to treat lupus. The patients in the German clinical trial are still being studied to see if or when the lupus returns to their bodies, Littlejohn said. The Cleveland Clinic trial is still only in its first of three phases and is only being offered to patients who have not responded to other lupus medications, Littlejohn said.
"This is a very promising therapy. It's really exciting, but we still have a lot to learn about it before we can provide it to a general lupus population," Littlejohn said.
Butler will continue to have regular appointments for the next several years. As of her most recent appointment, on July 8, her symptoms and activity markers have continued to improve. Butler said she hopes to eventually be in remission.
"It's not just a new medication that might help manage symptoms. If the trial does for me what it's done for everyone that's gotten it so far, it means remission for the first time," said Butler. "I've never been in remission before, so this would be life-changing."