Drugmaker doubles recall of infant ibuprofen sold at Walmart, CVS and Family Dollar stores
Tris Pharma is doubling a previous recall of infant ibuprofen sold at Walmart, CVS Pharmacy and Family Dollar stores nationwide because the popular pain and fever medicine may contain overly high concentrations of the drug.
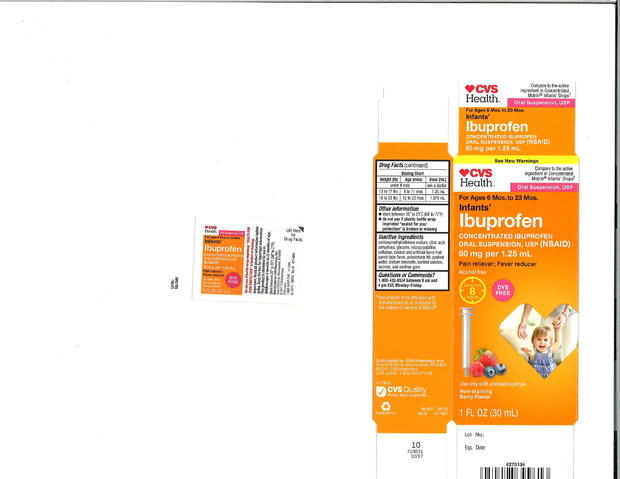
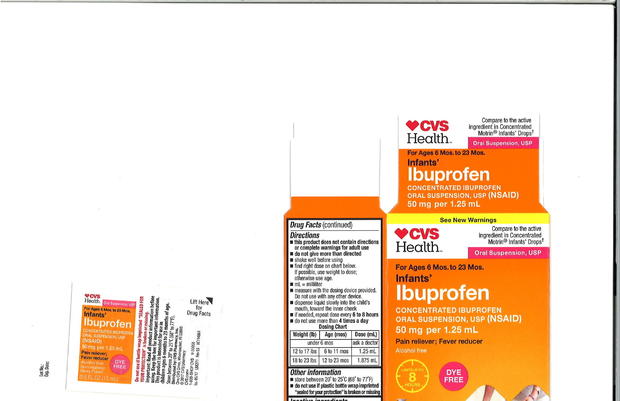
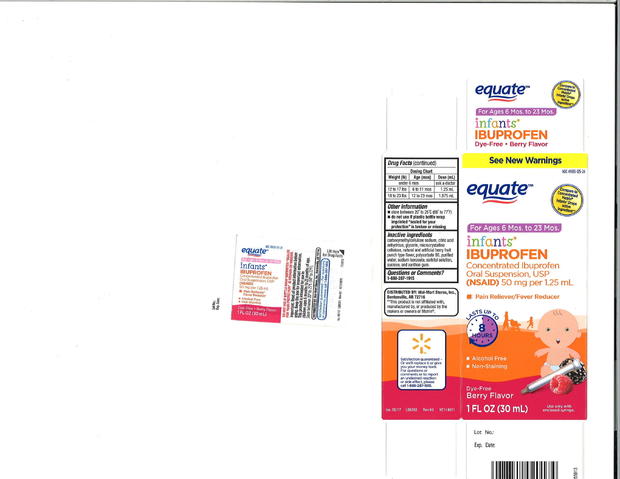
Packaged under the three retailers' private-label brands, the label on the recalled product says it contains 50 mg of ibuprofen per 1.25 ml. Some units involved in the recall were found to contain Ibuprofen levels as high as 10 percent above the specified limit, the company said in a statement.
Infants already susceptible to the adverse effects of ibuprofen may be at a slightly higher risk if given medication from one of the recalled bottles. Tris said there is a "remote possibility" an elevated level of ibuprofen could lead to permanent kidney damage in babies. Other possible adverse health effects include nausea, vomiting, stomach pain and diarrhea, as well as tinnitus, headache and stomach bleeding.
The latest recall expands on one first announced by the Monmouth Junction, New Jersey, drug maker in early December. In both cases, Tris Pharma said it hadn't received any reports of related health problems.
Founded in 2000, the privately held company researches, develops, manufactures and markets over-the-counter, Rx branded, and specialty generic products in the U.S.
The recalled products sold at Walmart had expiration dates of February 2019, April 2019 and August 2019, while those made for CVS and Family Dollar expire in August 2019 and December 2019. The recalled product was packaged in 0.5 ounces and 1 ounce bottles and labeled "Infants' Ibuprofen Concentrated Oral Suspension" (see product images below).