Glenmark Generics birth control pills recalled over packaging error
(CBS/AP) Birth control pills made by drugmaker Glenmark Generics are being recalled because some pills were in the wrong order, the company announced Friday. It's the second recall of generic birth control pills announced this month over faulty packaging.
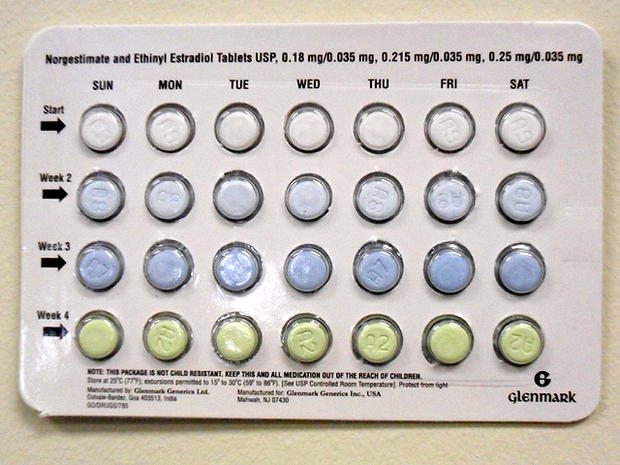
Glenmark, which is based in India, did not say how many pills or packages are being recalled, but said the recall affects seven lots. Glenmark said the pills were distributed between Sept. 21 and Dec. 30. The ingredients of the pills are norgestimate and ethinyl estradiol.
In some packages, blisters of pills were rotated so they were not in the proper sequence. The error also made the lot number and expiration date harder to see, and company said the recall applies to any blister package for which the lot number and expiration date are not visible. At this time, there remains sufficient supply of unaffected lots in the marketplace to support demand, the company said.
To see which lots were recalled, visit the FDA's website.
A month's supply of birth control pills contains three different medications that are each taken one a day for a week, and a week's worth of inert pills. If the pills are taken out of order, they may not work, which could result in an unwanted pregnancy. Glenmark's pills were intended to be taken in this order: seven off-white tablets, seven light blue tablets and seven blue tablets, followed by a week of green placebo pills (pictured above). When taken properly, birth control pills are nearly 100 percent effective. The packaging defects do not pose any immediate health risks, the company said.
This is the second major recall of birth control pills this month over packaging problems. Pfizer Inc., the largest drugmaker in the world, announced a similar recall on Feb. 1, HealthPop reported. The company pulled about 1 million packets of Lo/Ovral-28 and its generic equivalent off the market, although it announced that only about 30 packets had the pills out of order.