First treatment for accelerated aging disease progeria has promising results
(CBS News) The first experimental drug for the treatment of progeria has brought hope for the families and people affected by the accelerated aging disease.
Progeria patients who used a farnesyl transferase inhibitors (FTI) called lonafarnib were able to improve their cardiovascular health and increase their weight gain, problems usually associated with the condition. The drug was initially slated by manufacture Merck to treat brain cancer, but was found ineffective against that disease.
Rare Disease Day spotlights rare conditions and need for treatments
Pictures: Progeria: First black child with rare aging disease
Clues about how we age found in genetic disorder
"This is a fantastic first step," says Leslie Gordon, medical director for the Progeria Research Foundation, a physician at Boston Children's Hospital and Brown University and the mother of a child with progeria, told NPR.
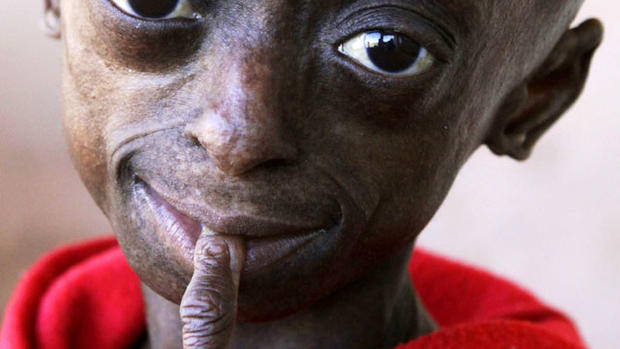
Though there are different varieties of progeria, the term progeria usually refers to Hutchinson-Gilford Progeria Syndrome or HGPS, which is a genetic condition that results in premature aging. According to the Progeria Research Foundation, the disease is caused by a gene mutation. Typically, the LMNA gene creates the Lamin A protein, which helps keep the cell nucleus together. But, in children with progeria the gene problem creates defective Lamin A proteins, which makes their cells unstable.
Patients with progeria experience premature aging, which leads to growth failure, loss of body fat and hair, aged looking-skin, stiffness of joints, bone loss, hip dislocation, general heart disease or arteriosclerosis, cardiovascular disease and stroke at an early age. Even though the Boston Globe estimates only 250 people worldwide have or had the disease, what makes it so tragic is it affects people at a young age and is 100 percent fatal. Accelerated aging begins at around 16 to 18 months of age, and by the time they are 10, the children look about 80. Patients on average die of heart disease complications by the age of 13.
Researchers discovered the culprit gene mutation in 2003 thanks to the Human Genome Product, and were able to find out the effect of defective Lamin A thanks to cell biology research. Then, Robert Goldman, the Stephen Walter Ranson Professor and Chair at Northwestern University School of Medicine, and other nuclear lamin researchers discovered that a greasy tag molecule called farnesyl affects the structure of the nuclear envelope and stops genetic messages that would have otherwise created regular cell growth.
Using this information, researchers tried a farnesyl transferase inhibitors (FTI) named lonafarnib to see if it could treat the conditions of progeria on 26 children. Although one child died five months into the trial, 25 were able to have at least two years of therapy. Results showed that the medication stopped blood vessel blockages that usually lead to heart related problems. Arterial stiffness went down as much as 35 percent. Bone loss slowed, with skeletal rigidity improving to normal levels. Children were able to gain weight as well, a problem considering that progeria patients normally weight about one-third of what their peers do. Nine patients were able to more than double their rate of weight gain thanks to increased muscle and bone mass.
"While they did not generate the kind of weight gain that was originally put forward as the primary endpoint, the effects on the cardiovascular system - which is in many ways the most serious component of the disease - were gratifying," Dr. Francis S. Collins, a director of the National Institutes of Health who was not involved with the study, said to the Boston Globe.
Because of the length of the study, it is unknown if the treatment can prevent an early death. But, some parents observed energy level changes, including the mother of one child who told the Wall Street Journal that her son, 16-year-old Devin Scullion, was eating and sleeping better when taking the treatment, and had increased energy. A second trial, which involves lonafarnib and two other drugs, is currently underway at Boston's Children's Hospital. The researchers are also working on U.S. Food and Drug Administration approval for the medication.
"There's no question: We got up to bat and for sure made it on base, which is the first time in this disease anyone has done anything that in any way, shape, or form altered the natural history of this disease," Dr. Mark Kieran, a neuro-oncologist at the Dana-Farber/Children's Hospital Cancer Center who headed the trial, said to the Boston Globe.
The results were published in the Proceedings of the National Academy of Sciences on Sept. 24, 2012.