Coronavirus survivor Andy Cohen fights for change in blood plasma donation rules for gay men
TV host Andy Cohen is a coronavirus survivor. Now he's in a new fight over a policy preventing him from helping other patients just because he's gay. FDA guidelines currently prevent men from donating blood plasma if they've had sex with another man in the past three months.
Cohen, the host of "Watch What Happens Live with Andy Cohen" on Bravo, told "CBS This Morning" on Monday that he was "disappointed" that, because he is gay, he cannot give blood plasma, as survivors may have antibodies which could help fight the virus.
"I've known in the past about the fact that gay men cannot donate blood," Cohen said. "But I think we're in an unusual situation right now. We're in a war against a disease that we don't know a lot about, and there's an urgent need for the antibody that is in people like me who have survived coronavirus."
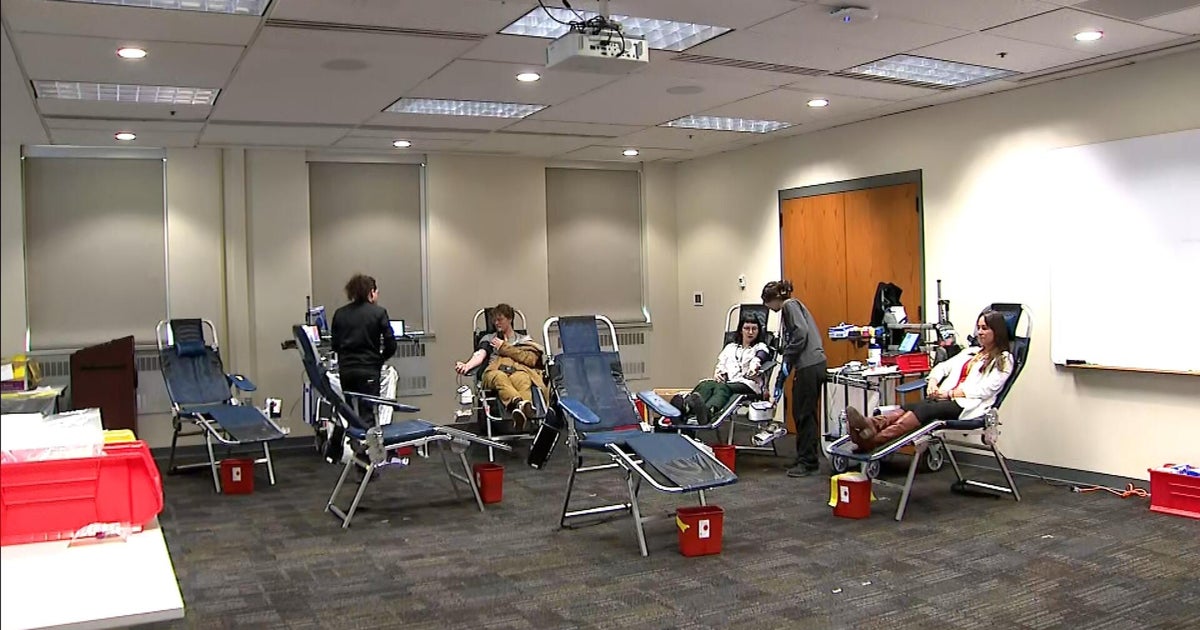
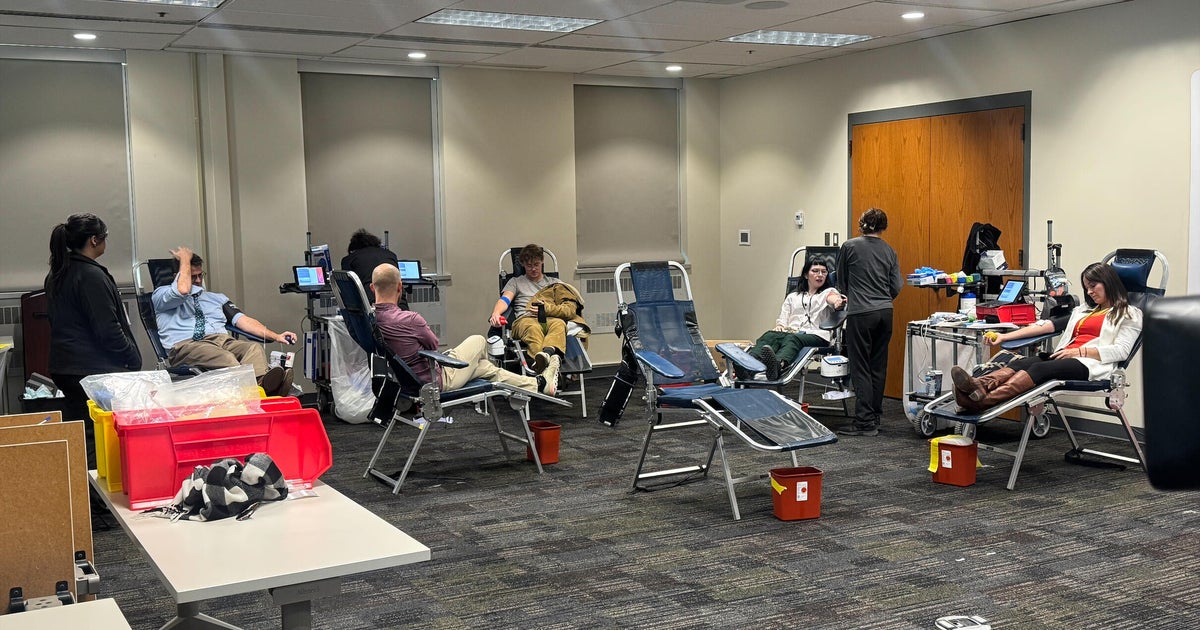
The Red Cross recently announced that there have been 150,000 fewer blood donations since the beginning of the coronavirus outbreak. Cohen told "CBS This Morning" co-host Gayle King, "There is an urgent need, and a special urgent need for the blood that I have. I want to help.
"I think that the rules should be looked at, again, by the FDA. There have been great strides in testing for HIV since these rules were enacted. You can get an HIV test in 20 minutes. They, I know, screen the blood again, a second time after you donate it to make sure there's not HIV in the blood. So, I just think this needs another examination.
"We're in an unusual moment in time right now; there is a war going on," Cohen said. "We're all being asked to adapt in so many ways to new things in life, and we're doing it for the greater good as a community. I think there is something that the FDA should take a look at and say, 'How can we move forward?' This is an FDA thing, by the way; it's not a Red Cross thing, it's not the hospital that I applied to give my blood to. It all comes to the FDA."
Earlier this month, the FDA relaxed slightly its rule regarding blood donations due to the coronavirus pandemic, and is now allowing men to donate blood three months after having sex with another man, instead of after 12 months.
The Food & Drug Administration told "CBS This Morning" that they are also working on a pilot study with 2,000 men to determine whether an individual risk assessment questionnaire would be as effective as their current policy. [Read the full statement below.]
"Listen, if they're working on something, that makes me happy," Cohen told King. "I don't know how long that's going to take. I just want to give my blood, you know what I mean? I want to help. And so that's what I'm looking to do."
He added, "If you're someone who's watching and you survived coronavirus, go give blood. It is needed and appreciated."
On March 20, Cohen announced that he had tested positive for Coronavirus.
King asked, "I just want to say you look good. Do you feel as good as you look? Because I'm wondering what the experience was like for you. You scared a lot of people, and there's so many different experiences about this."
He replied, "There are. I had a great friend who had coronavirus, and he was about four days ahead of me in terms of the virus working its way through his system. So, I was talking to him every day and finding out where he was. I had a less severe case than he did. But I feel good. I feel strong. And now I'm just trying to help and pitch in and do what I can just as a citizen."
King also asked Cohen about his son, Benjamin, recently voted by People Magazine as the "Cutest Baby Alive."
"Cutest baby alive, Gayle!"
He described being separated from Benjamin: "It stunk, man. He was down the hall. I was in my room basically shut out from civilization for 13 days. And you know, it was a killer. I could hear him, I was watching him on the nanny cam. Thank God I have an incredible caretaker for him who really did yeoman's work. But it was not great, man."
"How are you finding happiness during these times," King asked.
"I am finding happiness actually by leaning in to hosting 'Watch What Happens Live' from home every night," Cohen said. "We're having a great time, and it's making me happy that my staff is employed and engaged, and we're getting to take people's minds off of the serious issues of the day and, like, lean into some fun and cocktails at the end of the night every night on Bravo."
Full Statement from the Food & Drug Administration to "CBS This Morning":
"The FDA remains committed to gathering the scientific data that support donor deferral policies that are non-exclusive while helping to ensure a high level of blood safety. For example, the FDA is committed to considering alternatives to the time-based deferral for men who have sex with men by generating the scientific evidence that will support an effective individual risk assessment-based blood donor questionnaire. In this regard, to investigate the scientific validity of such an approach, the FDA is working to commence a pilot study that will enroll about 2000 men who have sex with men and who would be willing to donate blood. This study, being conducted at community health centers in key locations across the United States, could generate data that will help the FDA determine if a donor questionnaire based on individual risk assessment would be as effective as time-based deferrals in reducing the risk of HIV.
"A summary of FDA's rationale for the change to a three-month deferral period for male donors who have sex with another man, can be found in FDA's guidance, "Revised Recommendations for Reducing the Risk of Human Immunodeficiency Virus Transmission by Blood and Blood Products" (issued by the Center for Biologics Evaluation and Research).
"Both the United Kingdom and Canada have moved to a three-month deferral period for men who have sex with men, and to date, there have been no reports from these countries suggesting safety concerns following the implementation of this change.
"As noted above, FDA remains committed to further investigating individual risk assessment as an alternative to time-based deferrals. FDA has done modeling showing that removal of the deferral entirely without implementation of some alternative way of identifying those at increased risk of recent HIV infection could lead to decreased safety of the blood supply."