First-Of-Its-Kind Cancer Treatment Developed By Santa Monica Company Helps Save Lives
SANTA MONICA (CBSLA) — An Orange County woman given only months to live is now cancer free, thanks to a one-of-a-kind treatment developed by a Santa Monica company.
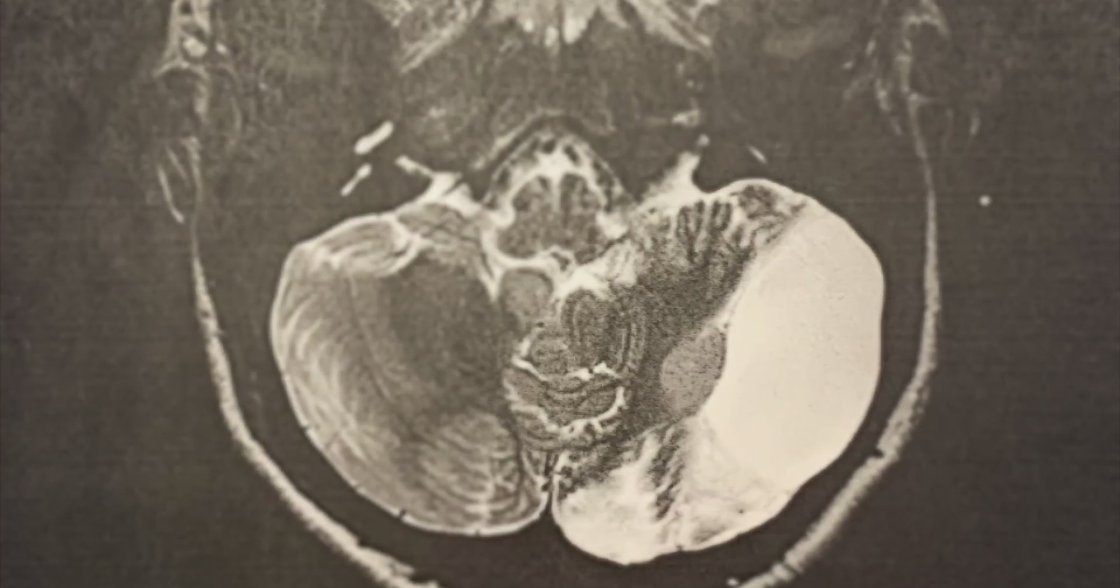
In 2015, Denise Delatorre was diagnosed with aggressive lymphoma. Chemotherapy failed to work. She was given six months to live.
"That day I went home and told my son I was going to die. I actually pulled him out of work and told him that I wasn't going to make it," Delatorre recalled as tears welled up in her eyes.
But then the Laguna Beach mother read about a study at UCLA involving a unique immunotherapy called CAR-T cell therapy. She became the first patient to be involved in the clinical trial.
"Your body is a miraculous thing. It's supposed to heal itself. Our immune systems are meant to heal disease," Delatorre said.
And that is exactly what biopharmaceutical company, Kite Pharma, hopes to do with its development of the first-of-its-kind therapy, which uses a patient's own cells to fight cancer.
T-cells are extracted from the patient's blood, modified to fight and kill the cancer cells and then injected back into the patient's body.
John Timmerman is a UCLA oncologist and member of the Jonsson Comprehensive Cancer Center tumor immunology program. He studied five patients. including Delatorre.
"These are the patient's own cells that have now been re-engineered to fight cancer in a way that the normal T-cells cannot do," UCLA Timmerman explained. "These are patients that have failed all other therapies and have really had few options up until now."
So far, the results are promising. Kite Pharma studied 101 patients across the country.
After an average of nearly nine months, 82 percent saw their tumors shrink during the study while 39 percent, including Delatorre, were in complete remission.
"I had no hope when I entered in clinical trials. So I feel extremely blessed," Delatorre said.
Before treatment, she had more than 30 tumors. After the treatment, those tumors were gone, a scan showed.
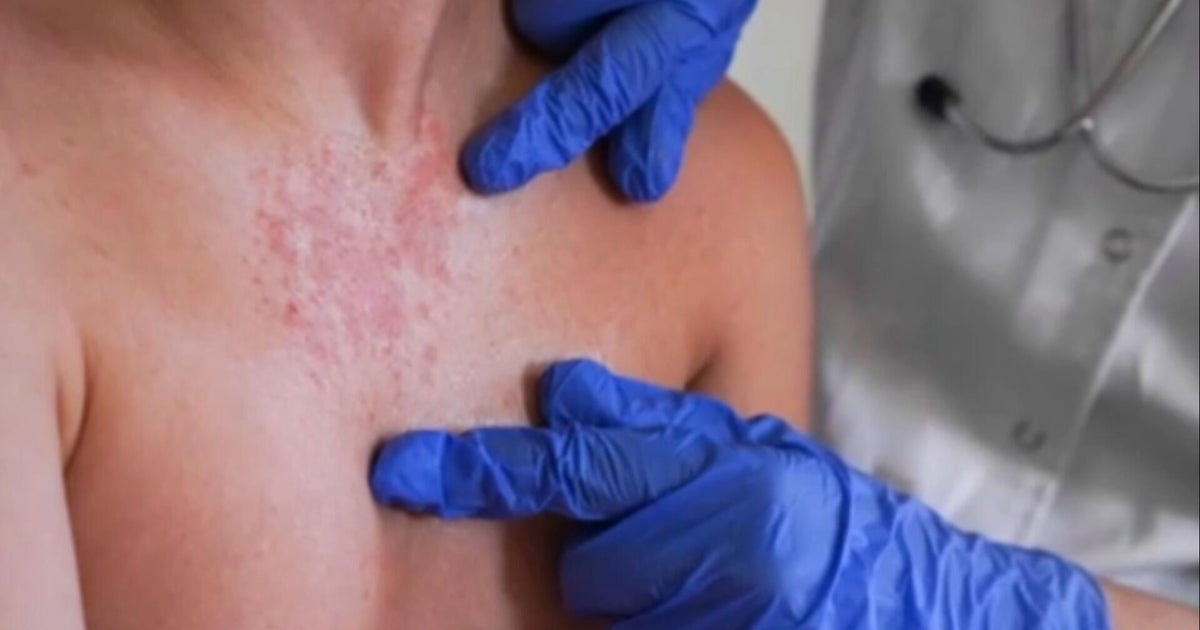
However, there are risks. Some of the patients developed a condition in which their immune system overreacted to the therapy. Two of those patients died.
Since then, doctors said they have adapted. "There's been a learning curve associated with using this therapy in myself and with other doctors around the country," Timmerman said. "I think we know how to use this therapy much more safely now."
The hope is that even if cancer re-emerges in Delatorre, her engineered cells will continue to fight.
"The T-cells should just pop back up and take care of that cancer, so I believe that is the true miracle of this," the 57-year-old said.
In remission now for a couple of months, Delatorre said was finally feeling like herself again.
"I started buying more clothes, and I started a new job. I'm starting to live my life again," she said with a big smile.
Kite Pharma hopes to have the CAR-T cell therapy approved by the FDA by November.