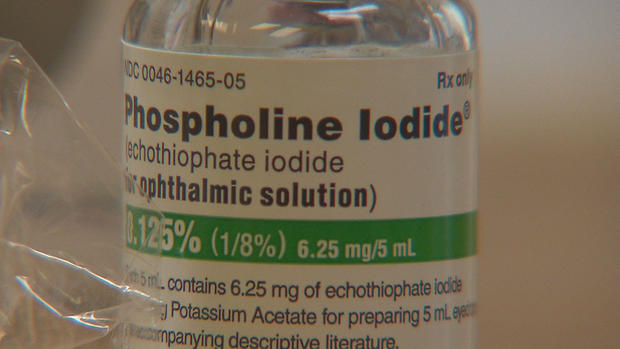I-Team: Parents Say Boy Could Go Blind After Pfizer Discontinued 'Miracle Drug'
BOSTON (CBS) - Tate Decker is a brave four-year-old. The little guy has had nine surgeries just to be able to see. "The next one would be a different one and would be more invasive," his dad Joe Decker said. "So we are trying to avoid it."
Tate who has an older and a twin brother, was born with childhood glaucoma complicated by cataract surgery. It is a disease with no cure, but it can be treated.
In Tate's case, eye drops made by Pfizer are helping to control the pressure in his right eye without the need for more surgery and the risk of blindness.
"It works," Tate's mom Holly Decker said. "It's our miracle drug."
Dr. David Walton has been treating glaucoma patients for more than 40 years, and is Tate's physician. He says the drops are, "uniquely successful for these patients who have this special kind of glaucoma." That glaucoma is called infantile aphakia glaucoma.
But recently Pfizer, which has made billions on its COVID-19 vaccine, told families it will no longer be manufacturing the Phospholine Iodide drops.
"It's heartbreaking, absolutely heartbreaking for us," Holly said. "And I know other families are going through the same thing." She is concerned for Tate and the next generation of patients who too will need this medicine.
In a statement, Pfizer said in part:
"Over the years, the P.I. supply chain has become increasingly unstable.... Use of P.I. has declined over the years, and based on recent prescription data, we estimate that the number of patients using the medicine is approximately 100 in the U.S."
Dr. David Walton said, "Pfizer has not been very helpful frankly." He tells the I-Team he has dozens of patients using the Pfizer drops, all managing to avoid surgery. Dr. Walton says he contacted Pfizer, after finding two smaller drug companies that were willing to make the drops. Pfizer didn't respond. "Pfizer could do much, much more to be responsible before just checking out and saying we not going to provide it," Dr. Walton said.
"We are scared. We need to get those drops," Holly said. "This is when he is learning how to read, learning letters, colors, shapes. It's a whole different ballgame when you are struggling to see."
Pfizer discontinued the medicine as of May 1, Leaving families scrambling to find a supply. Still hopeful that another company will step up and manufacture the drops.
Full Statement From Pfizer to I-Team:
We understand that some patients, caregivers, and ophthalmology scientific organizations are disappointed about Pfizer's decision to discontinue Phospholine Iodide (P.I.). We did not come to this decision lightly and made every effort to inform those impacted as soon as the decision was made to allow time to seek the best alternative treatment options. Over the years, the P.I. supply chain has become increasingly unstable, and glaucoma treatment has significantly evolved over the past 50+ years.
Pfizer relies on a complex chain of external partners to produce P.I., and this has led to multiple stock-outs. Despite our best efforts to find a solution, we are no longer able to guarantee continued supply of this medicine. Additionally, several alternative treatments are now available for glaucoma (used alone or in combination) including eye drops (prostaglandins, beta-blockers, cholinergics, alpha adrenergic inhibitors, carbonic anhydrase inhibitors, Rho kinase inhibitors, nitric oxides), laser treatment, and surgery. Among the eye drops, prostaglandins and beta-blockers (alone or in combination) are most frequently used.
P.I. is no longer routinely used in general glaucoma care, but a limited number of ophthalmologists continue to use it for select patients as an add-on therapy. Use of P.I. has declined over the years, and based on recent prescription data, we estimate that the number of patients using the medicine is approximately 100 in the U.S.
The decision to exit manufacture and supply was taken only after all avenues for improvement and alternate sourcing had been explored without success. It is important to note that the manufacturer does not have control of the full supply chain. Pfizer relied on a complex supply chain involving multiple suppliers of active ingredients, excipients, specialist packaging materials, technologies, final formulation and packaging. It's also important to note that the active ingredient for the formulation is toxic in nature requiring specialist handling and transportation.